Potential evaluation of central nervous system anti-depressant activity of Cleome rutidosperma in mice
Abstract
Introduction: This investigation was carried out to analyze the central nervous system (CNS) depressant effect of the plant Cleome rutidosperma extract, after it was found to have been used by the local people in the Philippine for that purpose.
Methods: In this study presented below, the CNS depressant effects of the extract was evaluated in in vivo mice models; using the standard procedures of Open field, Hole cross and Thiopental sodium induced sleeping time tests.
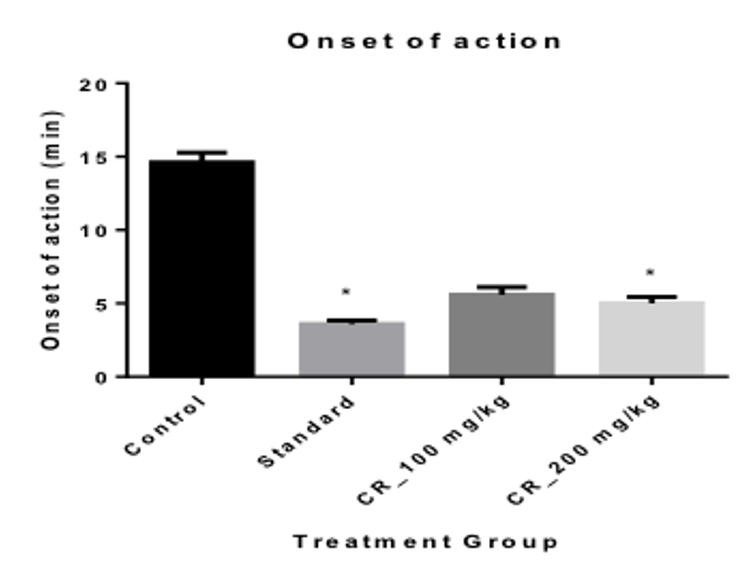
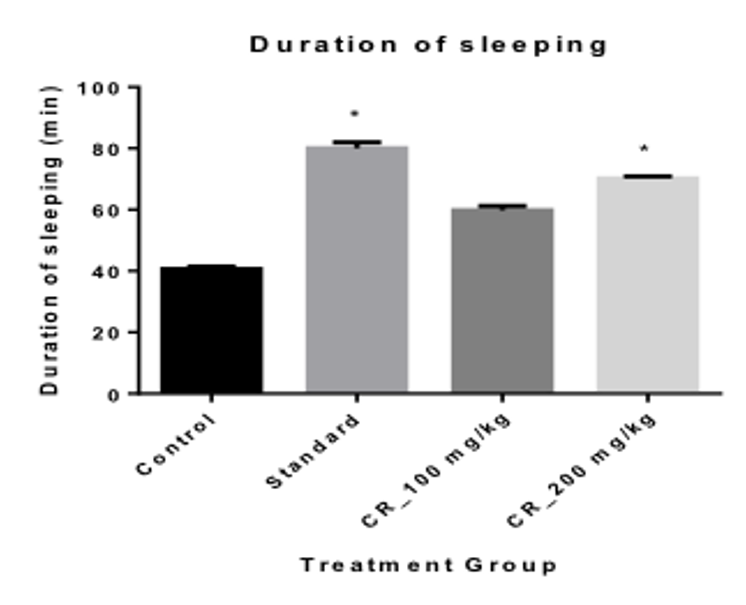
Results: Using two test extracts at a concentration of 100 and 200 mg/kg, it was seen that the extracts showed significant (p< 0.01) dose dependent suppression of motor activity in both open field and hole cross test, 4.67 ± 0.68** and 3.00 ± 0.45**, respectively at 200 mg/kg. It also showed significant (p< 0.01) decrease in the time for the onset of sleep (5.00 ± 0.45 at 200 mg/kg); and an increase in sleeping duration (70.20 ± 0.66 at 200 mg/kg), when compared with the positive control Thiopental sodium.
Conclusion: Overall, the study demonstrates that the extracts used, showed promising CNS depressant effect. Further study needs to be carried out on the extract to isolate the active constituent, so that it can be assessed for therapeutic use.
Introduction
Anxiety and depression are two most prevalent psychiatric diseases that have been reported in these days. More than 20% of the mature population suffers from these diseases at some part of their lives Buller and Legrand, 2001 Titov et al., 2010 Yadav et al., 2008. Sedative and hypnotics drug classes are being used to cure anxiety and these drugs produces relaxation by reducing onset of sleep time as well as increasing duration of sleep Katzung et al., 2011. Thus, present demand has increased use of these drugs largely, to treat different psychiatric disorders like, anxiety and insomnia. However, continuous use of this sedative-hypnotic therapeutics may cause some serious side effects ranging from respiratory and immune system disorders to damaging the cognitive nerve function, and can also cause physical dependency Dhawan et al., 2003. The development of a new sedative-hypnotic drug is therefore needed with fewer side effects and hence, a promising approach to prevent different psychiatric disorders.
Cleome rutidosperma, is a flowering plant species, which belongs to the genus Cleome of the family Cleomaceae, and is commonly known as Fringed Spider Flower or Purple Cleome. This species is a kind of invasive weed that are found everywhere in the low, wet tropical regions of Asia and Oceania continent. It is a very common weed of lawns. Crude methanol, chloroform and petroleum ether extracts of Cleome rutidosperma shows significant analgesic effect and depressed locomotor activity substantially compared to control treatment with chlorpromazine Bose et al., 2004.
Based on this finding, the experiment was carried out to further assess the extracts potentiality on the Central Nervous System (CNS). We found that the extract of Cleome rutidosperma, significantly reduced the locomotor activity and motor coordination in mice. Furthermore, pretreatment with this extract potentiated thiopental sodium-induced hypnosis in mice by decreasing the onset of sleep and prolonging sleeping duration. Therefore, our findings strongly support the sedative and hypnotic activities of Cleome rutidosperma extract and suggest that, upon successful isolation of the active molecule from the extract, it can be used in future as treatment of different psychiatric disorders including insomnia.
Methods
Extract preparation
C. rutidosperma DC was collected from Mirpur area, Dhaka, Bangladesh in October, 2013. The specimen of the plant was identified by the taxonomists of National Herbarium, Dhaka, Bangladesh (Accession No. 38625). The leaves of the plant was washed, dried, and mashed to powder. About 500 g of the dried plant powder was dissolved in 1500 ml of methanol and stirred rigorously for the following three days. Then the filtrate was collected using a sterilized cotton filter and dried in a rotary evaporator. Finally, 37.67 g (yield 7.53%) of plant extract was obtained and stored at freezing condition for future use.
Animal model sampling
20-25 g (male) of Swiss Albino mice were obtained from the International Center for Diarrheal Disease Research, Bangladesh (ICDDR, b). Then, under the standard environmental conditions, (temperature: 24.0 ± 1.00C; relative humidity: 55-65%; 12 h light and dark cycle), these animals were housed. Mice were provided with the food and fresh water, ad libitum. According to the Ethical Principles and Guidelines for Scientific Experiments on Animals (1995) formulated by The Swiss Academy of Medical Sciences and the Swiss Academy of Sciences, treatment for all the experimental animals were designed. The protocol was examined, and then accepted by the Ethics Committee of School of Health and Life Sciences, North South University, Dhaka-1229, Bangladesh.
Acute oral toxicity testing
Mice were grouped into the control and three test groups (n = 5). The test groups received MECR (methanolic extract of C. rutidosperma) orally at the doses of 1500, 2000, and 3000 mg/kg body weight. Then they caged separately and provided free excess to food and water. They were observed for the next three days Imam and Sumi, 2014 Walker et al., 2008 for possible changes in the behavior, allergic reactions (like skin rash, itching) and mortality.
Open field test
This test was carried out in an apparatus having a floor of about half square meter in area and surrounded by a wall of 50 cm in height Gupta et al., 1971. The floor consisted of small squares alternately, colored in black and white. Four test groups containing five mice in each, was classified as control, positive control and two test groups. The control group was treated with vehicle (1% Tween 80 in water) and the positive control was treated with diazepam (1 mg/ kg). The test groups were treated with 100 mg/kg and 200 mg/kg of the extract, respectively.
The number of squares visited by the mice was counted and noted for an interval of 3 minutes; before and after 30, 60, 90 and 120 minutes of the oral administration of the vehicle, diazepam and the test extracts.
Hole cross test
This test was carried out in a closed chamber, surrounded by wooden walls measuring 30 cm × 20 cm × 14 cm, with no roof top Takagi et al., 1971. A fixed wooden partition was placed in the middle of the chamber, which divided the chamber into two parts. The partition had a hole cut in it, measuring 3.5 cm and a hole height of 7.5cm.
Four test groups, with each group containing five mice each were selected for this test. The groups were classified as control, positive control and two test groups; with the test groups receiving 100 mg/kg and 200 mg/kg of the extract. The control and the positive control group received vehicle (1% Tween 80 in water) and diazepam (1 mg/kg), respectively.
The number of times it took for each mice to cross in between the chambers via the hole, was measured for an interval of 3 minutes; before treatment and 30, 60, 90 and 120 minutes after the oral administration of the vehicle, diazepam and the doses of 100 and 200 mg/kg of the extract .
Thiopental sodium induced sleeping time
For this experiment, four groups with five mice in each group were taken, similar to the previous experiments. The groups were classified as control, positive control and two test groups; with the test groups receiving 100 mg/kg and 200 mg/kg of the test extract. The control and the positive control group received vehicle (1% Tween 80 in water) and diazepam (1 mg/kg), respectively Ferrini et al., 1974.
Thirty minutes after the treatment, thiopental sodium was administered intraperitoneally at a dose of 20 mg/kg to the mice in each group and they were placed in separate chambers. The latent period (time between thiopental sodium administration and loss of righting reflex) and the duration of sleep (time between the loss and recovery of righting reflex) was observed for each mouse. The onset and duration of sleep were recorded for the four groups.
Statistical Analysis
The results have been portrayed as Mean ± SEM. The one-way ANOVA test along with Dunnett’s post hoc test had been used for the inspection of data using GraphPad Prism 6 software. p < 0.05-0.001 were considered as statistically significant.
Results
Open field test
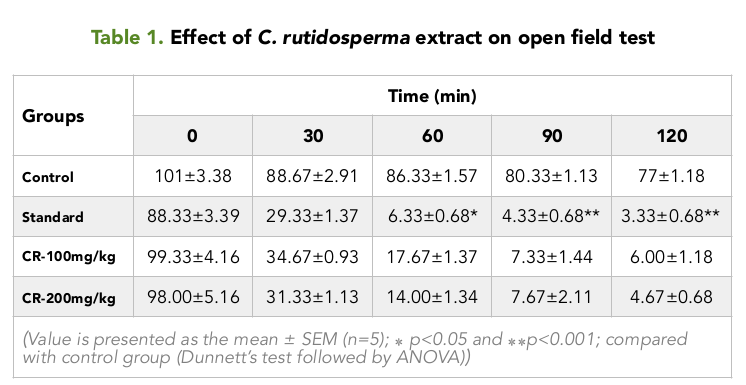
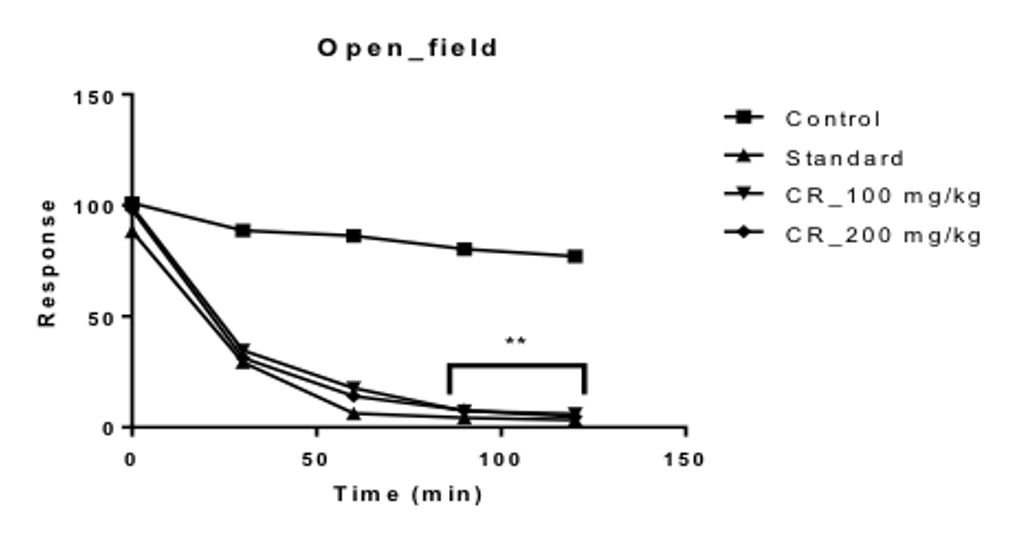
In the Open field test, it was observed that, the squares visited by the different groups of mice; before the treatment were 101.00, 88.33, 99.33 and 98.00 for the control, positive control and the test groups treated with 100 and 200 mg/kg Table 1 Figure 1 respectively.
As time passed on, it was seen that, the number of squares visited by the mice of the different groups, decreased over time for an interval of 2 hours. For the control group, the numbers of squares visited were nearly constant, from values ranging from the highest value of 88.67 to the lowest value of 77.00. Table 1 Figure 1
For the 100 and 200 mg/kg test groups, it was seen that the test group treated with 200 mg/kg of the extract showed the lower number of squares visited by the mice over time, compared to the test group treated with 100 mg/kg .The highest and lowest value for the 200 mg/kg treated group were 31.33 and 4.67 during the two hour period, respectively. For the 100 mg/kg treated group, it was 34.67 and 6.00 Table 1 Figure 1 respectively. At all times, the values for the positive control group was the lowest among the four groups, with the highest being 29.33 at 30 min and the lowest being 3.33 at 120 min Table 1 Figure 1 for an interval of 2 hours.
Hole cross test
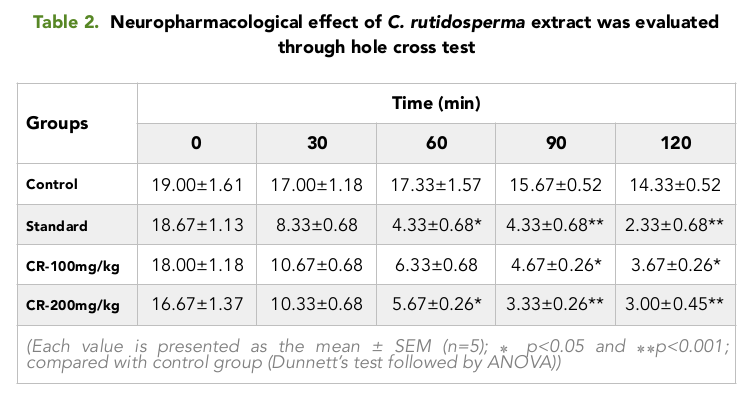
Like the Open field test, it was observed in the hole test that, the number of times taken by the mice to cross between the chambers, decreased gradually as time passed on for a period of 2 hour Table 2 Figure 2 . For all the groups, the numbers of crossing between the chambers were higher in the pretreatment period, where no extract or positive control was given to the mice.
As time passed on from 30 min to 120 min, it was seen that, the mice belonging to the positive control group showed the highest change in the number of crossing between the chambers with the lowest value of 2.33 at 120 min whereas, the test group treated with 200 mg/kg showed a considerable decrease in the number of crossing with a value of 3.00 at 120 min Table 2 Figure 2 , when compared with the Control group and the test group treated with 100 mg/kg.
Thiopental sodium induced sleeping time
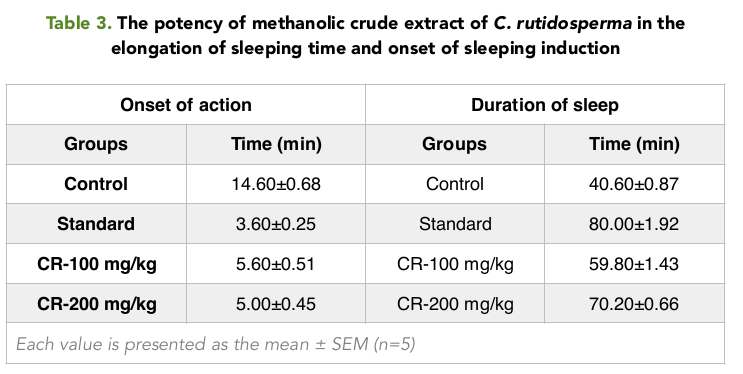
When measuring the Onset of sleep, it was seen that the onset was most rapid in the positive control group with only 3.60 mins Table 3 . The test Group 2 had the lowest onset of sleep time of 5 mins when compared with the Control group and the test Group 1 When measuring the duration of sleeping time, it was seen that, the duration of sleeping time was the highest in the positive control group with 80 mins; the next being the mice in the group 2 and group 1 with a duration of 70.20 and 59.80 Table 3 Figure 4 respectively.
Discussion
This research has demonstrated that the administration of different doses (100 and 200 mg/kg body weight) of methanol extract of C. rutidosperma leaves, showed strong sedative and anxiolytic properties. Both doses potentiated sleep during the thiopental sodium induced sleeping time tests. "Thiopental" that is a hypnotic agent, and basically cause hypnosis by potentiating GABA mediated postsynaptic inhibition through allosteric modification of GABA receptors at a proper dose. Substances that consist of CNS depressant activity either decrease the onset or prolong the duration of sleep or dose both Hasan et al., 2009 Nyeem et al., 2007. GABA-benzodiazepine receptors are the most abundant inhibitory receptor Braestrup and Squires, 1977 system in the CNS and binding of a benzodiazepine agonist to its binding site results in an increase in chloride ion flux Trofimiuk et al., 2005 that in turns hyperpolarizes the postsynaptic membrane at a lower threshold of spike generation. This mechanism of GABA agonists made them available for generation of hypnosis in the treatment of anxiety. In addition, the hole cross and open field methods carried out to test the locomotor activity, which shows both doses of methanol extract from the leaves of C. rutidosperma decreased the frequency and the amplitude of movements. Since, locomotor activity is a measurement of the level of excitability of the CNS Mansur et al., 1980, this decrease in spontaneous motor activity could be attributed to the sedative effect of the plant extracts (Rakotonirina et al., 2001). Both doses significantly, decreased locomotion in mice. The locomotor activity lowering effect was evident from the 2nd observation of (30 min) and continued up to the 5th observation period at (120 min) of the hole cross and the open field test. The results were also dose dependent and statistically significant (p<0.001).
The research has examined some neuropharmacological activities of methanolic crude extracts of C. rutidosperma leaves. The plant extracts possessed CNS depressant activity that has been shown by the decrease in exploratory behavior in mice. It also showed a marked sedative effect by the reduction in gross behavior and potentiation of thiopental induced sleeping time. Substances which possess CNS depressant activity either reduce the onset of sleep time or prolong the duration of sleep or dose both Nyeem et al., 2007. Moreover, the research on locomotor activity, as measured by hole cross and open field tests, showed that both extracts of the dried leaves of C. rutidosperma (100 and 200 mg/kg) reduced the incidence and the amplitude of movements. Since, locomotor activity is a measure of excitability of the CNS Mansur et al., 1980, this inhibition of continuous motor activity could be attributed to the sedative effect of the plant extracts Öztürk et al., 1996 Rakotonirina et al., 2001.
The medicinal effect of a plant usually results from the combination of a secondary metabolites present within it, through additive or synergistic action of several chemical compounds acts on single or multiple target sites associated with a physiological process Briskin, 2000. According to Kaufman et al., (1999) Kaufman et al., 1999, preliminary phytochemical analysis with this plant revealed the presence of alkaloids, tannins, glycosides, steroids, flavonoids and tannins. These secondary metabolites especially flavonoids individually or in combination with other phytochemicals, might account for the observed pharmacological effects exerted by this plant. However, many flavonoids were found to be ligands for the gamma aminobutyric acid type A (GABAa) receptors in the CNS, which led to the hypothesis that they acts like benzodiazepine molecules. Thus, the sedative and anxiolytic effects exhibited by the C. rutidosperma leaves extracts might be due to the interaction of flavonoids with the GABA/benzodiazepine receptor complex in brain Trofimiuk et al., 2005. This is findings supported by their behavioral effects in animal models of anxiety, sedation and convulsion Marder and Paladini, 2002. Electrophysiological experiments with flavone and flavanone derivatives have shown that some of them can modulate GABA-generated chloride currents, either positively or negatively. Due to the increased knowledge of the diversity of GABAa receptor sub-types, the number of studies with cloned receptors of defined subunit composition has risen recently and experiments with some natural and synthetic flavones and flavonones have shown that they can modulate gamma aminobutyric acid (GABA)- generated chloride currents, either positively or negatively Campbell et al., 2004 Goutman et al., 2003 Johnston, 2005 Kavvadias et al., 2004. Thus the decreased spontaneous motor activity could be attributed to the CNS depressant activity of the leaves of C. rutidosperma.
Conclusion
It can be concluded from the above experiment that, the extracts of Cleome rutidosperma possess significant sedative and hypnotic activities. With all the doses, used in the above experiment, it was clearly visible that, the effects were statistically significant. However, further studies must be carried out to isolate the active constituent from the extract; which is responsible for the CNS depressant activity, and hence investigate its potentiality for therapeutic use in future and to understand its molecular mechanisms responsible for that pharmacological activity.
Abbreviation
ANOVA: Analysis Of Variance; CNS: Central Nervous System; icddr’b: International Centre for Diarrheal Disease Research, Bangladesh
Ethical Approval
We declare that this study was done for the human beneficiary and under proper supervision of ethical committee of North South University. Nothing has been done that violate animal rights as this study involves use of a number of animals. We took our ethical consent from icddr’b (International Centre for Diarrheal Disease Research, Bangladesh) during collection of models for this experiment and also our institutional (Department of Pharmaceutical Sciences, North South University, Dhaka-1229, Bangladesh) authority approved us on this protocol to use animal model. And Prof. JMA Hannan led the ethical committee along with other intellectual body. They have inspected the protocol and estimated the animal use. After inspection, it is decided that the animal to be sacrificed in this protocol is justified. Then the committee issued an ethical approval stating that this protocol is eligible to use animal model for its purpose.
Authors contribution
SA and AC carried out the study design, participated in experiment. AC, FFH and SI contributed in the manuscript preparation. SA, SG and AU have done all data calculation and statistical analysis. MAHKB, MSK and MR have collected the plant and was involved in the herbarium of the plant. All the authors have scrutinises whole manuscript for gramatical and typographical mistake.
Funding
We declare that this project was accomplished by university funding and a partial funding came from involved researchers mentioned in the author list. No third party was included in this study by financial or any-other means.
References
-
A.
Bose,
V.
Saravanan,
N.
Karunanidhi,
J.
Gupta.
Analgesic and locomotor activity of extracts of cleome rutidosperma DC. Indian J Pharm Sci.
2004;
66
:
795-797
.
-
C.
Braestrup,
R.F.
Squires.
Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H) diazepam binding. Proceedings of the National Academy of Sciences.
1977;
74
:
3805-3809
.
-
D.P.
Briskin.
Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant physiology.
2000;
124
:
507-514
.
-
R.
Buller,
V.
Legrand.
Novel treatments for anxiety and depression: hurdles in bringing them to the market. Drug discovery today.
2001;
6
:
1220-1230
.
-
E.L.
Campbell,
M.
Chebib,
G.A.
Johnston.
The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA A receptors. Biochemical pharmacology.
2004;
68
:
1631-1638
.
-
K.
Dhawan,
S.
Dhawan,
S.
Chhabra.
Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: a non-habit forming anxiolytic. J Pharm Pharm Sci.
2003;
6
:
215-222
.
-
R.
Ferrini,
G.
Miragoli,
B.
Taccardi.
Neuro-pharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneimittel-Forschung.
1974;
24
:
2029-2032
.
-
J.D.
Goutman,
M.D.
Waxemberg,
F.
Doñate-Oliver,
P.E.
Pomata,
D.J.
Calvo.
Flavonoid modulation of ionic currents mediated by GABA A and GABA C receptors. European journal of pharmacology.
2003;
461
:
79-87
.
-
B.
Gupta,
P.
Dandiya,
M.
Gupta.
A psycho-pharmacological analysis of behaviour in rats. The Japanese Journal of Pharmacology.
1971;
21
:
293-298
.
-
R.
Hasan,
M.M.
Hossain,
R.
Akter,
M.
Jamila,
E.
Mazumder,
S.
Rahman.
Sedative and anxiolytic effects of different fractions of the Commelina benghalensis Linn. Drug Discov Ther.
2009;
3
:
221-227
.
-
M.Z.
Imam,
C.D.
Sumi.
Evaluation of antinociceptive activity of hydromethanol extract of Cyperus rotundus in mice. BMC complementary and alternative medicine.
2014;
14
:
1
.
-
G.A.
Johnston.
GABAA receptor channel pharmacology. Current pharmaceutical design.
2005;
11
:
1867-1885
.
-
B.G.
Katzung,
S.B.
Masters,
A.J.
Trevor.
Basic < clinical pharmacology. McGraw-Hill Medical New York.
2011
.
-
P.B.
Kaufman,
L.J.
Cseke,
S.
Warber,
J.A.
Duke,
H.L.
Brielmann.
Natural products from plants. CRC press Boca Raton^ eFL FL.
1999
.
-
D.
Kavvadias,
P.
Sand,
K.A.
Youdim,
M.Z.
Qaiser,
C.
Rice-Evans,
R.
Baur,
E.
Sigel,
W.D.
Rausch,
P.
Riederer,
P.
Schreier.
The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. British Journal of Pharmacology.
2004;
142
:
811-820
.
-
R.
Mansur,
W.
Martz,
E.
Carlini.
Effects of acute and chronic administration of Cannabis satis and (-) 9-transtetrahydrocannabinaol on the behaviour of rats in open field arena. Psychopharmacol.
1980;
2
:
5-7
.
-
M.
Marder,
A.C.
Paladini.
GABA-A-receptor ligands of flavonoid structure. Current Topics in Medicinal Chemistry.
2002;
2
:
853-867
.
-
M.
Nyeem,
M.
Alam,
M.
Awal,
M.
Mostofa,
S.
Uddin,
N.
Islam,
R.
Rouf.
CNS depressant effect of the crude ethanolic extract of the flowering tops of Rosa Damascena. 2007
.
-
Y.
Öztürk,
S.
Aydin,
R.
Beis,
K.
Başer,
H.
Berberoĝlu.
Effects of Hypericum perforatum L. and Hypericum calycinum L. extracts on the central nervous system in mice. Phytomedicine.
1996;
3
:
139-146
.
-
V.S.
Rakotonirina,
E.N.
Bum,
A.
Rakotonirina,
M.
Bopelet.
Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia.
2001;
72
:
22-29
.
-
K.
Takagi,
M.
WATANABE,
H.
SAITO.
Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. The Japanese Journal of Pharmacology.
1971;
21
:
797-810
.
-
N.
Titov,
G.
Andrews,
A.
Kemp,
E.
Robinson.
Characteristics of adults with anxiety or depression treated at an internet clinic: comparison with a national survey and an outpatient clinic. PloS one.
2010;
5
:
e10885
.
-
E.
Trofimiuk,
A.
Walesiuk,
J.J.
Braszko.
St John's wort (Hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacological research.
2005;
51
:
239-246
.
-
C.I.
Walker,
G.
Trevisan,
M.F.
Rossato,
C.
Franciscato,
M.E.
Pereira,
J.
Ferreira,
M.P.
Manfron.
Antinociceptive activity of Mirabilis jalapa in mice. Journal of ethnopharmacology.
2008;
120
:
169-175
.
-
A.
Yadav,
L.
Kawale,
V.
Nade.
Effect of Morus alba L (mulberry) leaves on anxiety in mice. Indian journal of pharmacology.
2008;
40
:
32
.
Comments

Downloads
Article Details
Volume & Issue : Vol 3 No 10 (2016)
Page No.: 889-901
Published on: 2016-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4150 times
- Download PDF downloaded - 1456 times
- View Article downloaded - 23 times
 Biomedpress
Biomedpress