Abstract
Introduction: Osteoarthritis is a contributing factor for pain and loss of function of the knee. Osteoarthritis results in many damages to the knee; one of the most common damages that is difficult to recover is cartilage injury. This study aims to apply autologous osteochondral transplantation (OAT) under knee arthroscopy for the treatment of knee cartilage defects.
Methods: This was a prospective, descriptive and non-controlled study. Patients were diagnosed as having osteoarthritis, as confirmed by 1cm2 – 3cm2 cartilage defects. Arthroscopic OAT was performed on each patient. Treatment efficacy and safety were evaluated based on Lysholm, Oxford Knee Scores (OKS) and pain scales (VAS) after 3, 6, 12 and 18 months.
Results: From 3/2014 - 8/2016, 61 cases (54 women and 7 men) participated in the study. The average age was 55 ± 8 years old. Most cases had cartilage defects in the medial condyle. Results showed that Lysholm, OKS scores and VAS scales improved after 12 months of treatment. Of the cases, 33 of 61 were followed out to 18 months; these patients showed improvement in knee function and pain scores. There was 1 case with incomplete matching between the plug and receiving site and 1 case with a broken plug. At the final stage of monitoring, there were no patients who experienced complications, such as broken instruments or fracture of condyle, nor who experienced early postoperative complications, such as infection and bleeding.
Conclusion: Autologous osteochondral transplantation via arthroscopy is a safe and promising method for the treatment of knee cartilage defects in patients with average osteoarthritis.
Introduction
Articular cartilage lesions cause pain and decreased mobility, affecting the working capacity and quality of life. To date, cartilage defects have been treated by different strategies, including debridement and lavage, microfracture, osteochondral autograft transplantation, osteochondral allograft transplantation, autologous chondrocyte implantation, and stem cell transplantation. Debridement and lavage are procedures of the oldest technique and typically reserved for low- demand older patients with small lesions (<2 to 3 cm2) Bert and Maschka, 1989Federico and Reider, 1997Freedman et al., 2004Owens et al., 2002. Current research has suggested that the best candidates for debridement and lavage are those who suffer from mechanical symptoms (Moseley et al., 2002). Meanwhile, for patients with small to moderate sized lesions (1 to 5 cm2), microfracture is a suitable treatment. The microfracture process helps stimulate fibrocartilage in-growth into the chondral defect to cover the underlying bone Freedman et al., 2004Gill and Macgillivray, 2001Steadman et al., 2003. The procedure is performed by creating tiny fractures in the subchondral bone plate.
Moreover, osteochondral autograft plugs have been investigated as a means to restore cartilage defects. Osteochondral autograft transplantation has been most commonly applied to treat symptomatic lesions Freedman et al., 2004Hangody et al., 2001. The greatest advantage of osteochondral autografts is the use of live hyaline cartilage. This technique results in cartilage that is most similar to the injured cartilage. However, this technique also has disadvantages, namely donor site morbidity (pain and new cartilage defect), technical difficulty and risk of cartilage or bone collapse.
Fresh osteochondral allograft transplantation entails the implantation of a cadaveric osteochondral graft into the cartilage defect Aubin et al., 2001Bugbee, 2000Garrett, 1994. This technique can be used for large articular cartilage defects (from 3 cm2 up to an entire hemicondyle). The major advantage of osteochondral allografts is the ability to replace large osteochondral defects in a single-stage procedure.
Currently, autologous cultured chondrocyte implantation has also been explored for the treatment of cartilage defects. In this technique, a small piece of cartilage is harvested arthroscopically. Chondrocytes from the sample are isolated and grown expanded in culture over several weeks. In the next step, millions of autologous cultured cartilage cells are suspended in a solution of fibrin glue and later implanted into cartilage defects Peterson et al., 2000. This technique is usually considered for intermediate to high-demand patients who have failed arthroscopic debridement or microfracture Brittberg et al., 1994Chu et al., 1999Gillogly et al., 1998.
Stem cell transplantation is currently another promising therapy for osteoarthritis and cartilage defects. Some recent studies have shown that autologous adipose stem cell transplantation can improve osteoarthritis Bui et al., 2014. Combination of stem cell transplantation and microfracture have also proven to be better than microfracture alone Nguyen et al., 2016. However, similar to osteochondral allograft transplantation and autologous chondrocyte implantation, stem cell transplantation is expensive but yields promising results in clinical trials.
In this study, we aim to investigate the application of osteochondral transplantation to treat cartilage defects of osteoarthritic knee.
Methods
Inclusion criteria
From March 2014 to August 2016, 61 patients (54 women and 7 men) were enrolled in our study; all had degenerative knee of grades III or IV (classified by Outerbridge), with cartilage lesions with an area of 1- 3 cm2 on the weight-bearing surface of the femoral condylar. All patients who participated in our study underwent arthroscopic osteochondral autologous transplantation. The mean age of the patients was 55 ± 8 years old.
Exclusion criteria
All patients with any of the following characteristics were excluded from our study: joint space ≤ 2mm, varus/valgus alignment > 50, knee stiffness, other joint diseases (e.g. rheumatoid arthritis, inflammation and neoplasm), and joint damage (e.g. caused by systemic diseases). Most patients had 1 or 2 plugs of osteochondral graft and 1 patient had 3 plugs. Moreover, 60 patients had lesions on the medial femoral condyle and 1 had lesions on the lateral femoral condyle (LFC). The mean size of cartilage defects was 1.54 cm2. Patients were given clinical and functional evaluations pre-operatively and at 3, 6, and 12 months post-operation using the Lysholm, OKS and VAS scales.
Surgical procedure
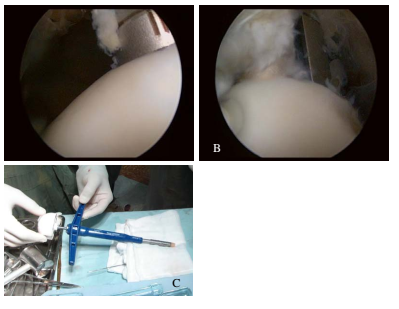
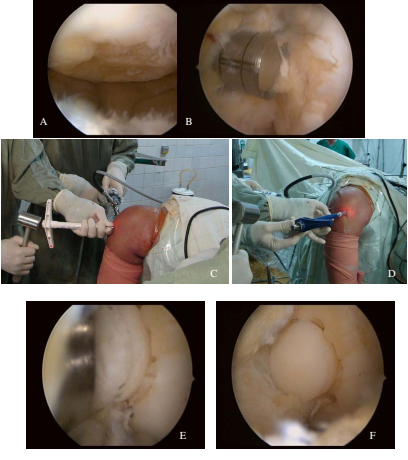
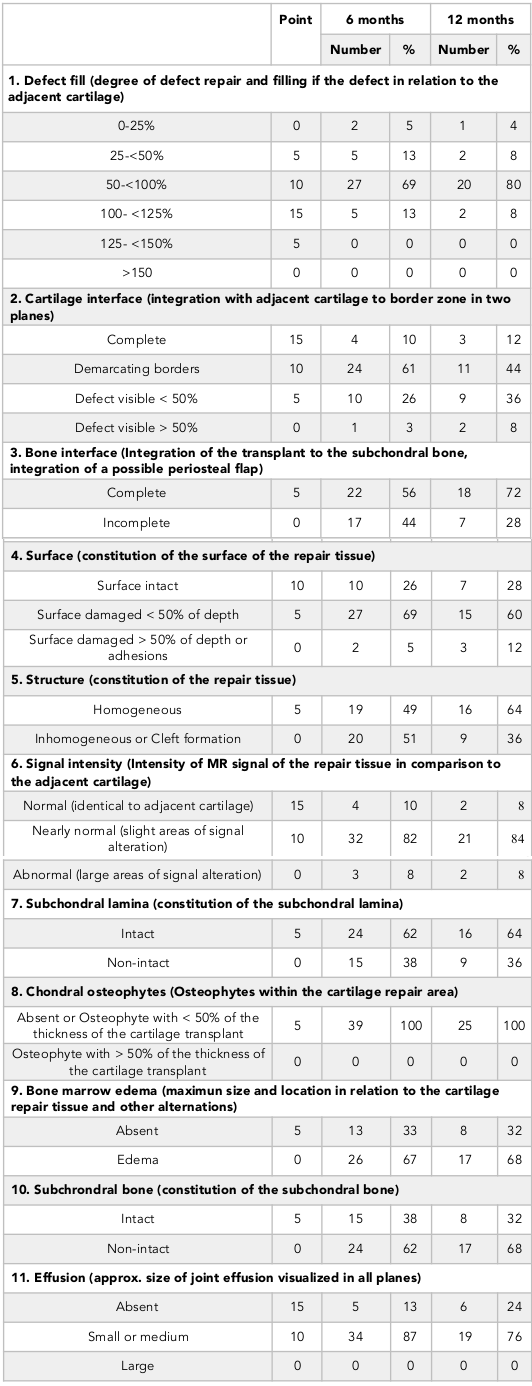
Surgery was performed under arthroscopy. The location of the defect was determined. Remnants of residual cartilage were removed from the defect. The size of the defect was measured. The osteochondral grafts were later removed from the donor site on the superior-lateral aspect of the LFC or trochlea and transferred into the cartilage defect. The length of plug was at least 15 mm and similar to the recipient site depth ( Figure 1 , Figure 2 ).
Post-operative rehabilitation
The knee was passively mobilized on the second post-operative day. Touch-down weight bearing with crutches was allowed after 6 weeks, and the patient could then move gradually toward full weight bearing (at about 8 weeks).
Statistical Analysis
All continuous data were calculated as mean values and standard deviation of the mean. The Kolmogorov Smirnov test was performed to assess the normal distribution of the continuous variables. The normal distribution values were compared using t-tests. Non-normal distribution values or small numbers were compared using the Wilcoxon signed-rank-test and Mann-Whitney U test. Pearson correlation coefficients were used to determine the correlation between MOCART score and cartilage defect size, and between MOCART score and clinical outcomes. Confidence level for all analyses was set at p <0.05. The statistical data was processed using the SPSS 16.0 software (IBM Corp., Armonk, NY, USA).
Results
Changes in Lysholm, OKS and VAS scores
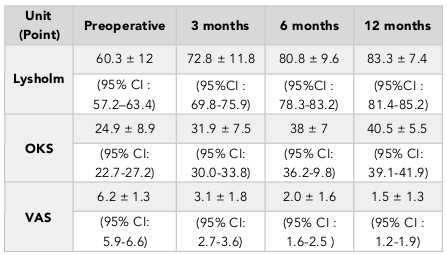
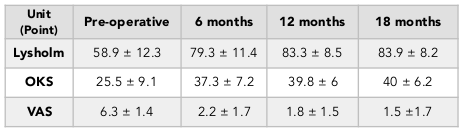
The results showed an improvement in Lysholm, OKS and VAS scores at 3, 6, and 12 months after surgery. Specifically, the OKS score increased significantly from 24.9 ± 8.9 to 40.5 ± 5.5 afab8er 12 months (paired t-test, p<0.001). Moreover, the VAS score decreased significantly from 6.2 ± 1.3 to 1.5 ± 1.3 after 12 months (paired t-test, p<0.001) ( Table 1 ). In 33 patients who were followed out to 18 months, the same trend was observed. In fact, there was no significant difference when comparing the 12-month-follow up results with the 18-month results (paired t-test, p>0.05), thus demonstrating that improved outcomes were maintained out to 18 months post-operation ( Table 2 ). The window in which patients felt improvement of symptoms was at about 3.7 months (1 to 7 months) after surgery.
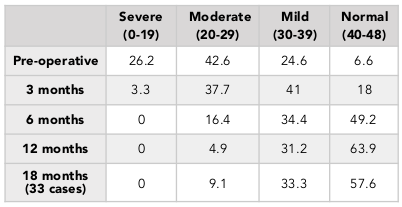
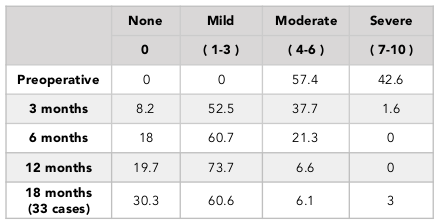
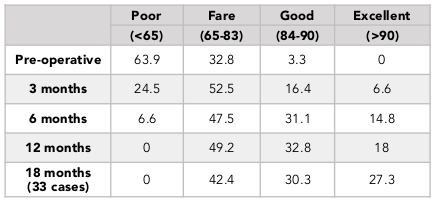
The percentage of patients with normal or mild knee arthritis, based on the OKS scores, increased from 31.2% (pre-operation) to 95.1% (at 12 months post-operation) ( Table 4 ). We also found that the percentage patients with no or mild pain, based on the VAS scores, went from 0% (pre-operation) to 93.4% (at 12 months post-operation) ( Table 5 ). The percentage of patients with pre-operative good knee function, based on the Lysholm scores, was 3.3% (pre-operation) and 32.8% (at 12 months post-operation) and there was no patient with poor function (63.9% preoperative) ( Table 3 ). Similar results was found in the 18-month follow-up group ( Table 2 ).
There are 35 cases with one-plug OAT and 25 cases with double-plug OAT. Both groups showed improvement in function and VAS scores at 12 months post operation (paired t-test, p<0.001; Wilcoxon signed-rank test, p>0.05). Moreover there was no significant difference between those two groups at any follow-up time (Mann-Whitney U, p>0.05) ( Table 7 ).
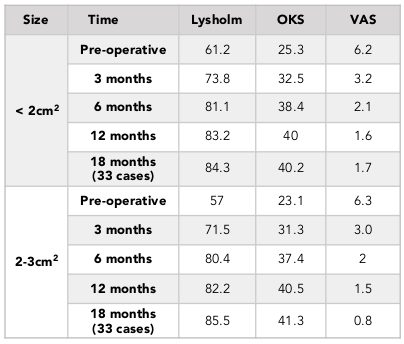
When divided into two groups according to the size of lesion, (2 – 3 cm2 group and <2 cm2 group), we found that there was no significant difference in any of the scales at all follow-up times (Mann-Whitney U, p>0.05) ( Table 6 ). Moreover, the size of defect had no correlation with clinical outcomes (p > 0.05). In the 18-month follow-up group, there were also no significant difference when comparing outcomes of single-plug group and double-plug group (Mann-Whitney U, p > 0.05) between the 2-3 cm2 and < 2 cm2 groups (Mann-Whitney U, p > 0.05) ( Table 6 and Table 7 ). There was no plug migration into the joint space, as assessed by clinical evaluation and knee X-ray after surgery.
MRI was performed for 39 patients at 6 months post-operation and for 25 patients at 12 months post-operation. For all cases, the MOCART scores were calculated, as well as assessment of integration of grafts into the receiving site, and intact cartilage surface. The mean MOCART was 61.8 ± 18 (95% CI: 55.9 – 67.7) at 6 months post-operation, and 62.4 ± 16 (95% CI: 55.5 – 69.3) at 12 months post-operation ( Table 8 ).
The rate of complete defect fill (100 – 125%) was 13% after 6 months and 8% after 12 months; the rate of partial cartilage defect fill (50 – 100%) was 69% after 6 months and 80% after 12 months ( Table 8 ). At 6 months after surgery, the rate of complete integration of plug into subchondral bone was 56%, and 72% after 12 months. Eighteen patients had MRI performed at both 6 and 12 months post-operation; there was an observed increase of 3D MOCART, from 61.7 ± 18 to 64.4 ± 14.7, though the difference was not significant (Wilcoxon, p = 0.341).
Imaging results
There was no correlation between the size of lesion and MOCART score at 6 and 12 months post-operation (p=0.15 and p=0.263, respectively) ( Figure 3 ). We also found no correlation between MOCART score (or its variables) and clinical outcome scores (Lysholm, OKS and VAS) (p> 0.05 for all).
Twenty single-plug cases and eighteen double-plug cases received an MRI evaluation at 6 months post-operation. The mean MOCART score for the single-plug group was 66.7 ± 17.9 and for the double-plug group was 56.4 ± 17.7; however, the difference was not significant (Mann-Whitney U, p=0.112). Similarly, the MRI results after 12 months for the 12 single-plug cases and 12 double-plug cases revealed no significant difference in MOCART score between the groups (Mann-Whitney U, p = 0.630).
Complications
We did not observe any complications during the operation, such as breakage of instrument, fracture of femoral condyle, and anterior cruciate ligament (ACL) or posterior cruciate ligament (PCL) attachment injury. There was one case of incomplete matching between plug and receiving site and one case of broken plug. Both instances entailed replacement of a new plug. None of the 61 experienced any early post-operative complications, such as infection, hemorrhaging or migration of plug.
Discussion
Hangody et al. recommended the use of OAT only for patients <40 years of age; there have been relative contraindications in patients ranging from 40-50 years of age, and contraindications in those >50 years of age Hangody et al., 2001. Kish et al., 1999 as well as Marcacci et al., 2005 have also reported better results in younger patients. However, Chow et al. have found that age is not a factor which limits the procedure; old people with chondral defects and a stable knee joint can achieve good results Chow et al., 2004. In our study, we also found in patients with a mean age of 55 ± 8 years old, clinical results as well as pain scores improved at 12 and 18 months post-operation. In a recent study, mosaicplasty for treatment of cartilage defects demonstrated promising results. In a multi-center study, Hangody et al. showed that mosaicplasty was better than other cartilage repair methods, including debridement, subchondral penetration and abrasion arthroplasty Hangody et al., 2001. Similarly, Krych et al. saw better activity levels after osteochondral autograft transfer mosaicplasty than after microfracture Krych et al., 2012.
Several other studies have also demonstrated improved results after mid-term and long-term follow-up. Randomized studies with a control group have been performed. Horas et al. Horas et al., 2003 and Dozin et al. Dozin et al., 2005 concluded that the clinical outcome of mosaicplasty was equivalent to autologous chondrocyte implantation, with a high rate of hyaline cartilage. However, after early- stage promising results, Solheim et al. Solheim et al., 2010 showed that there is a gradual reduction of efficacy after 10 to 14 years of follow-up; 40% of the 73 cases had poor outcome, and good outcome was often seen in younger patients with defect size <3 cm2. In a study of 52 patients at 37 months follow-up, Jakob et al. found that the method was limited by the defect size and the number of plugs taken at the donor site Jakob et al., 2002. Marcacci et al. studied 30 patients and confirmed better outcome was associated with small defect size and with only 1-3 plugs Marcacci et al., 2005.
In our study, after the 12 month follow-up period, the percentage with good and excellent Lysholm score was 50.8%. The percentage having knee with normal or mild inflammation on the OKS scale was 95.1%, and the rate of mild pain or no pain on the VAS scale was 93%. Although the Lysholm scale results was lower than previous studies, the results of the VAS scores and OKS scores were equivalent to what other authors, such as Marcacci et al., 2005 and Jakob et al., 2002 have published. The reason for the lower Lysholm scale scores in this study may be due to the fact that the patients in our study included those with knee osteoarthritis.
Osteochondral autograft transplantation for isolated cartilage defects with < 2-3 cm2 lesion area in young people requiring high activity is nothing controversial. In our study, osteochondral autograft transplantation for grade III/IV cartilage defects with 1-3 cm2 lesion area on the weight‐bearing surfaces of femoral condylar in older adults with osteoarthritis is an expanded indication to delay knee replacement surgery. Initial results showed good results with lesion area of ≤ 3 cm2 at 12 months post-operation and similar results in the 18 months post-operative group. In studies by Hangody et al., the authors only performed OAT for cartilage defect sizes from 1-4 cm2, although it can be applied as a temporary method for 8 cm2 cartilage defects Hangody et al., 2001.
In this study, we compared the results in two groups of lesion defects: <2 cm2 and 2-3 cm2. We saw good results in both groups; there was no correlation between lesion size and clinical results. Marcacci et al. observed better results with smaller-sized lesions Marcacci et al., 2005, but other authors have found Jakob et al., 2002, as we did too, that there are no statistically significant correlation between clinical outcome and lesion size.
With the development of diagnostic imaging devices, MRI provides not only a non-invasive means to diagnose cartilage lesions but also a reliable tool for monitoring and evaluating results of articular cartilage lesion treatment. In particular, 3D MOCART is a good scale and most often used for evaluating results of OAT by MRI Marlovits et al., 2006Marlovits et al., 2004. 3D MOCART scale assesses many variables, including: degree of repair filling, integration of the cartilage repair tissue to the border zone, structure of the surface, structure of the whole repair tissue, and signal intensity. Thus, the scale can be used to evaluate the effectiveness, success or failure of treatment.
Rate of complete defect fill after 1 year according to research by Zak et al. is 50% Zak et al., 2014. In our study, this rate was only 8%; most cases (80%) had defect fill from 50% to <100%. We found intact surface rate of cartilage after 1 year to be 28%; Zak group’s found it to be 70% Zak et al., 2014. This difference may be due to parameters in other studies which are not seen in osteoarthritis patients. The majority of patients in our study are older and osteoarthritic thus MRI results after 1 year were worse. However, the rate of complete bone interface was 72% and we did not have any complete delamination case, meaning that all plugs were stable and in place.
The average MOCART score after 12 months in our study was 62.4 points, not too much lower than 75 points in Zak et al.’s study Zak et al., 2014 and Krusche-Mandl et al.’s study Krusche-Mandl et al., 2012. We also did not find a correlation between MOCART score and function scores or VAS scores.
Concerning the correlation between MOCART score and clinical outcome, Krusche-Mandl et al. did not find any correlation between MOCART score and Lysholm, IKDC or VAS scores Krusche-Mandl et al., 2012. Tetta et al. also found that there is only a correlation with the IKDC scale but not with the Tegner scale Tetta et al., 2010. A recent meta-analysis study also found that there is not enough evidence to confirm a correlation between morphological results of MRI and clinical outcome Wakitani et al., 2002.
Ensuring the matching between plugs and the receiving site to create a smooth cartilage surface is a challenge in the OAT technique. Chow et al. showed that harvesting and transplanting of osteochondral plugs should be perpendicular to cartilage surface; in fact, wrong angle placement will reduce efficacy Chow et al., 2004. Marcacci et al. also agreed with this assessment after 3 of their cases failed and were related to the surface matching problem Marcacci et al., 2005. Hangody and Fules also emphasized the importance of matching between plugs and the receiving location Hangody et al., 2001. Therefore, in our study, we carefully performed the OAT procedure, and only 1 case without matching after transplantation had to be replaced with another plug.
Conclusion
OAT procedure under arthroscopy was investigated as a treatment for osteoarthritis patients with grade III/IV cartilage defects (1-3 cm2 lesion area) and showed promising initial results. OAT is an accepted and trusted method in the treatment of 1-3 cm2 cartilage defects, helping to delay knee replacement surgery. There is no correlation between 3D MOCART and functional outcome, or to post-operative pain score. OAT under arthroscopy may be a promising procedure for the treatment of knee articular cartilage defects since it is a minimally invasive, low-priced, and one-stage procedure which yields some efficacy and few complications.
List of Abbreviations
OAT: autologous osteochondral transplantation
OKS : Oxford Knee Score
LFC : Lateral femoral condyle
MOCART :Magnetic resonance observation of cartilage repair tissue
Ethical approval and informed consent
All patients gave informed consent and the studies were approved by the Committee of Department of Science and Technology, Ho Chi Minh city, Viet Nam.
Author Contribution
BHTK: collected, analyzed, data and wrote the manuscript; MTV, NDT, LTV, NPT: collected data, write the draft of manuscript; HNT: diagnosis, evaluated clinical scores; performed MRI evaluation. All authors approved this manuscript
References
-
P.
Aubin,
H.
Cheah,
A.
Davis,
A.
Gross.
Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clinical orthopaedics and related research.
2001;
391
:
S318-S327
.
-
J.M.
Bert,
K.
Maschka.
The arthroscopic treatment of unicompartmental gonarthrosis: a five-year follow-up study of abrasion arthroplasty plus arthroscopic debridement and arthroscopic debridement alone. Arthroscopy: The Journal of Arthroscopic & Related Surgery.
1989;
5
:
25-32
.
-
M.
Brittberg,
A.
Lindahl,
A.
Nilsson,
C.
Ohlsson,
O.
Isaksson,
L.
Peterson.
Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New england journal of medicine.
1994;
331
:
889-895
.
-
W.D.
Bugbee.
Fresh osteochondral allografting. Operative Techniques in Sports Medicine.
2000;
8
:
158-162
.
-
K.H.-T.
Bui,
T.D.
Duong,
N.T.
Nguyen,
T.D.
Nguyen,
V.T.
Le,
V.T.
Mai,
N.L.-C.
Phan,
D.M.
Le,
N.K.
Phan,
P.
Van Pham.
Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomedical Research and Therapy.
2014;
1
:
2-8
.
-
J.C.
Chow,
M.E.
Hantes,
J.B.
Houle,
C.G.
Zalavras.
Arthroscopic autogenous osteochondral transplantation for treating knee cartilage defects: a 2-to 5-year follow-up study. Arthroscopy: The Journal of Arthroscopic & Related Surgery.
2004;
20
:
681-690
.
-
C.R.
Chu,
F.R.
Convery,
W.H.
Akeson,
M.
Meyers,
D.
Amiel.
Articular Cartilage Transplantation: Clinical Results in the Knee. Clinical orthopaedics and related research.
1999;
360
:
159-168
.
-
B.
Dozin,
M.
Malpeli,
R.
Cancedda,
P.
Bruzzi,
S.
Calcagno,
L.
Molfetta,
F.
Priano,
E.
Kon,
M.
Marcacci.
Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clinical Journal of Sport Medicine.
2005;
15
:
220-226
.
-
D.J.
Federico,
B.
Reider.
Results of isolated patellar debridement for patellofemoral pain in patients with normal patellar alignment. The American journal of sports medicine.
1997;
25
:
663-669
.
-
K.B.
Freedman,
J.A.
Fox,
B.J.
Cole.
Knee cartilage: Diagnosis and decision making. Textbook of arthroscopy.
2004;
:
555-567
.
-
J.C.
Garrett.
Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clinical orthopaedics and related research.
1994;
303
:
33-37
.
-
T.J.
Gill,
J.D.
Macgillivray.
The technique of microfracture for the treatment of articular cartilage defects in the knee. Operative Techniques in Orthopaedics.
2001;
11
:
105-107
.
-
S.D.
Gillogly,
M.
Voight,
T.
Blackburn.
Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. Journal of Orthopaedic & Sports Physical Therapy.
1998;
28
:
241-251
.
-
L.
Hangody,
P.
Feczkó,
L.
Bartha,
G.
Bodó,
G.
Kish.
Mosaicplasty for the treatment of articular defects of the knee and ankle. Clinical orthopaedics and related research.
2001;
391
:
S328-S336
.
-
U.
Horas,
D.
Pelinkovic,
G.
Herr,
T.
Aigner,
R.
Schnettler.
Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. J Bone Joint Surg Am.
2003;
85
:
185-192
.
-
R.P.
Jakob,
T.
Franz,
E.
Gautier,
P.
Mainil-Varlet.
Autologous osteochondral grafting in the knee: indication, results, and reflections. Clinical orthopaedics and related research.
2002;
401
:
170-184
.
-
G.
Kish,
L.
Módis,
L.
Hangody.
Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in the athlete: rationale, indications, techniques, and results. Clinics in sports medicine.
1999;
18
:
45-66
.
-
I.
Krusche-Mandl,
B.
Schmitt,
L.
Zak,
S.
Apprich,
S.
Aldrian,
V.
Juras,
K.
Friedrich,
S.
Marlovits,
M.
Weber,
S.
Trattnig.
Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthritis and Cartilage.
2012;
20
:
357-363
.
-
A.J.
Krych,
H.W.
Harnly,
S.A.
Rodeo,
R.J.
Williams.
Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee. J Bone Joint Surg Am.
2012;
94
:
971-978
.
-
M.
Marcacci,
E.
Kon,
S.
Zaffagnini,
F.
Iacono,
M.P.
Neri,
A.
Vascellari,
A.
Visani,
A.
Russo.
Multiple osteochondral arthroscopic grafting (mosaicplasty) for cartilage defects of the knee: prospective study results at 2-year follow-up. Arthroscopy: The Journal of Arthroscopic & Related Surgery.
2005;
21
:
462-470
.
-
S.
Marlovits,
P.
Singer,
P.
Zeller,
I.
Mandl,
J.
Haller,
S.
Trattnig.
Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. European journal of radiology.
2006;
57
:
16-23
.
-
S.
Marlovits,
G.
Striessnig,
C.T.
Resinger,
S.M.
Aldrian,
V.
Vecsei,
H.
Imhof,
S.
Trattnig.
Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. European journal of radiology.
2004;
52
:
310-319
.
-
P.D.
Nguyen,
T.D.-X.
Tran,
H.T.-N.
Nguyen,
H.T.
Vu,
P.T.-B.
Le,
N.L.-C.
Phan,
N.B.
Vu,
N.K.
Phan,
P.
Van Pham.
Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Translational Medicine, sctm.
2016;
:
2016-0023
.
-
B.D.
Owens,
B.J.
Stickles,
P.
Balikian,
B.D.
Busconi.
Prospective analysis of radiofrequency versus mechanical debridement of isolated patellar chondral lesions. Arthroscopy: The Journal of Arthroscopic & Related Surgery.
2002;
18
:
151-155
.
-
L.
Peterson,
T.
Minas,
M.
Brittberg,
A.
Nilsson,
E.
Sjögren-Jansson,
A.
Lindahl.
Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clinical orthopaedics and related research.
2000;
374
:
212-234
.
-
E.
Solheim,
J.
Hegna,
J.
Øyen,
O.K.
Austgulen,
T.
Harlem,
T.
Strand.
Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. The Knee.
2010;
17
:
84-87
.
-
J.R.
Steadman,
K.K.
Briggs,
J.J.
Rodrigo,
M.S.
Kocher,
T.J.
Gill,
W.G.
Rodkey.
Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy: The Journal of Arthroscopic & Related Surgery.
2003;
19
:
477-484
.
-
C.
Tetta,
M.
Busacca,
A.
Moio,
R.
Rinaldi,
M.
Delcogliano,
E.
Kon,
G.
Filardo,
M.
Marcacci,
U.
Albisinni.
Knee osteochondral autologous transplantation: long-term MR findings and clinical correlations. European journal of radiology.
2010;
76
:
117-123
.
-
S.
Wakitani,
K.
Imoto,
T.
Yamamoto,
M.
Saito,
N.
Murata,
M.
Yoneda.
Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis and Cartilage.
2002;
10
:
199-206
.
-
L.
Zak,
I.
Krusche-Mandl,
S.
Aldrian,
S.
Trattnig,
S.
Marlovits.
Clinical and MRI evaluation of medium-to long-term results after autologous osteochondral transplantation (OCT) in the knee joint. Knee , Surgery, Sports Traumatology, Arthroscopy.
2014;
22
:
1288-1297
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 11 (2016)
Page No.: 985-1002
Published on: 2016-11-21
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 5023 times
- Download PDF downloaded - 1310 times
- View Article downloaded - 47 times
 Biomedpress
Biomedpress