Abstract
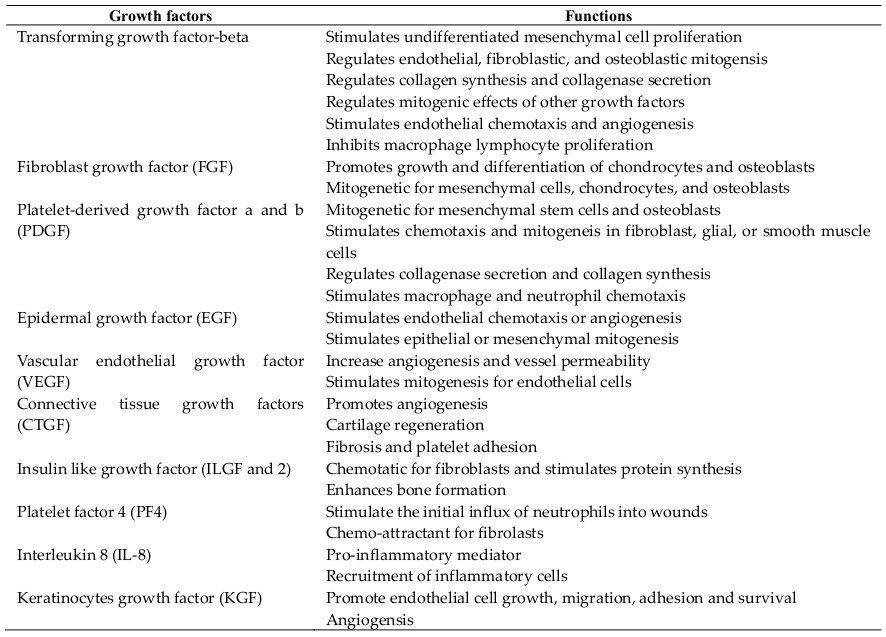
Platelet-rich plasma (PRP) contains at least seven growth factors including epidermal, platelet-derived, transforming, vascular endothelial, fibroblast, insulin-like and keratinocyte growth factor. The therapeutic effect of PRP occurs because of the high concentration of these growth factors compared with those found in normal plasma. In recent years, PRP is widely used across many clinical fields, especially in regenerative medicine. This review aimed at presenting an overview of the applications of PRP in regenerative medicine. The mechanisms of PRP effects on healing are also stated in this review.
Introduction
Platelets are fragments of megakaryocytes. They are considered as kinds of blood cells that hold important roles in blood coagulation and wound healing. In fact, platelets contain a great variety of growth factors regulating cytokines and initiating wound healing. Up to date, there are up to 300 proteins determined in the human platelets Coppinger et al., 2004. The main function of platelets is to prevent acute blood loss as well as repair vascular walls after injury. This function is related to the roles of the proteins and cytokines produced by platelets, and released to the injured site after platelets are activated. These proteins released by platelets create many important effects on many kinds of cells. Most created effects were recorded as cell proliferation, angiogenesis, cell migration, and tissue regeneration Anitua et al., 2004Nurden,2011. Particularly, some results showed that some peptides from platelets act as anti-microbial peptides Cieslik-Bielecka et al., 2012Drago et al., 2013Kraemer et al.,2011. Consequently, platelets, especially the platelet lysates, have become subjects of great interest to physicians and scientists in some fields for a long time. Platelet rich plasma (PRP) is a platelet-derived product, which has been in use for a long time with or without previous platelet activation. PRP has been in use since the 1970s and it became popular in the 1990s Marx, 2004. In recent years, PRP has been applied in disease treatments, especially as adjuvant for stem cell therapy. This review aimed at summarizing all applications of PRP in regenerative medicine and its role in stem cell therapy.
Healing process and platelet roles
Healing mechanism
Inflammation and blood coagulation are developments that trigger the healing process. After injury due to direct exposure of cells to physical, mechanical or chemical trauma, cells will face the apoptotic or necrotic condition. Accordingly, immune cells will infiltrate into the injured sites. Both apoptotic and necrotic cells and the immune cells will produce growth factors and cytokines that enhance the inflammatory process. These factors attract, more and more, the monocytes and neutrophils recruitment to injured sites. There are a lot of inflammatory factors released at the injured sites including IFN-gamma, interleukin-6, interleukin-1 and tumor necrosis factor – TNF-alpha. Some growth factors for healing process such as transforming growth factor (TGF), basic fibroblastic growth factor (bFGF), plateletderived growth factor (PDGF) and vascular endothelial growth factor (VEGF) are also recorded at injured sites.
After inflammation, new tissue formation occurs 2-10 days after injury. The new tissue formation results from two cellular biological processes which are cell proliferation and cell migration Eming et al., 2007. The topical proliferative phase of cells in injured cells is completely dependent on the roles of macrophage and platelets. They provide both matrix and cytokines for proliferation. Some matrix proteins are produced as fibrin, fibronectin, glycosaminoglycans and hyaluronic acid. They form a matrix for topical cells that adhere and proliferate. The proliferation is also pushed by cytokines such as FGF, EGF, HGF, KGF, TGF and PDGF. At injured cells, in almost all cases, progenitor cells are triggered by these cytokines to proliferate and differentiate into tissue fibroblasts. These fibroblasts proliferate and move through the extracellular matrix by binding fibronectin, vitronectin and fibrin, and responding with growth factor and cytokines at these injured sites. As a result, after 5-7 days, tissue fibroblasts become predominant cell kinds, following injury. These fibroblasts produce collagen, proteoglycans and other components, and proteins cover the injury and formation scars. New tissue formation is supported by angiogenesis which is performed at the same time with fibroblast proliferation.
Angiogenesis occurs as a result of endothelial cell migration and division. Angiogenesis forms new blood vessels that are essential in promoting blood flow to support the high metabolic activity in the newly deposited tissue. Angiogenesis is promoted by a combination of local stimulatory factors such as VEGF, and anti-angiogenic factors such as angiostatin, endostatin and thrombospondin. Local factors that stimulate angiogenesis include low oxygen tension, low pH and high lactate levels. Soluble mediators such as bFGF, HGF, TGFbeta and VEGF also stimulate endothelial cells to produce vessels. The new vessels allow the delivery of oxygen, nutrients and the removal of by-products.
Finally, the tissue enters into the last phase of healing in which granulation tissue matures into a scar. Collagen produced by fibroblast accumulation reaches a maximum at 2-3 weeks after injury, and the transition to remodeling begins. The most dramatic change occurs in the overall type, amount and organization of the collagen fibers, resulting in an increased tensile strength of the tissue. Initially, there is an increased deposition of collagen type III, also referred to as reticular collagen, that is gradually replaced by collagen type I. Collagen fibers are cross-linked by the lysyl oxidase enzyme, which is secreted by fibroblasts in the extracellular matrix. The normal adult 4:1 ratio of type I to type III collagen is restored during remodeling. Equilibrium is established as new collagen is formed, and collagen type III is degraded. The MMPs, collagenases, gelatinases and stromelysins control the degradation of extracellular matrix components to facilitate cell migration into the wound, angiogenesis and overall tissue remodeling.
Platelet and its role in healing process
Human blood contains 93% red blood cells, 6% platelets and 1% white blood cells. Platelets were firstly discovered by Alfred Donne in 1842 M., 1842. In 1980, platelets were considered as important blood particles involved in the healing of wounds. More than a decade later, platelets were determined to contribute to angiogenesis. In the healing process, platelets serve as rich sources of biological active proteins. Within the past three decades, we could activate the platelets and evaluate the roles of released proteins called growth factors.
Platelets contain intracellular organelles with two types of granules which are dense granules that contain the nonprotein substances secreted in response to platelet activation, including serotonin, ADP/ATP, histamine, dopamine and catecholamines; and granules that contain secreted proteins. Platelets contain at least 7 growth factors (secreted proteins) including transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF-AB and PDGF-BB), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and fibroblast growth factor (FGF)-2. TGF-β1 and PDGF stimulate proliferation of mesenchymal cells. TGF-β1 also stimulates extracellular matrix production, including collagen. VEGF and FGF- 2 are important for stimulating new blood vessel formation to bring nutrients and progenitor cells to the injury sites. Platelets were also known as resources of IGF-1.
Platelet rich plasma and regenerative medicine
Platelet rich plasma
Platelet-rich plasma (PRP) is defined as the fraction of plasma having a platelet concentration above baseline Marx, 2001. In fact, PRP must have a minimum increase of five times the normal concentration of platelets (1 million platelets per microliter of blood). Similar to platelets, PRP contains a pool of growth factors that hold important functions in regenerative medicine ( Table 1 ). To date, there have been some arguments about the deal concentration of platelets. Different from some previous studies, recent studies show that much higher concentration of platelets, compared to normal, does not show further enhancement of wound healing Marx,2004. The first blood bank PRP preparations began during the 1960s and became routine preparations through the 1970s.
At this concentration of platelet, PRP contains higher concentration growth factors than baseline. As a result, PRP dramatically stimulates the cell proliferation and migration. Proteins from artificial activated PRP exhibit a paracrine effect on different cell types including endothelial cells Freire et al., 2012, fibroblast Anitua et al., 2009Browning et al., 2012, osteoblasts Garcia-Martinez et al., 2012Graziani et al., 2006, chondrocytes Drengk et al., 2009van Buul et al., 2011, mesenchymal stem cells of different origins Cho et al., 2011Dohan Ehrenfest et al., 2010Mishra et al., 2009Pham et al., 2014Van Pham et al., 2013, tendon cells (Carofino et al., 2012; de Mos et al., 2008; Jo et al., 2012; Mazzocca et al., 2012) and myocytes Mazzocca et al.,2012.
At the present, PRP has been introduced in two forms, which are PRP and activated PRP (aPRP). Activated PRP is a PRP stimulated to form clot and release growth factors before applied in patients. In contrast to aPRP, PRP is known as plasma with enriched platelets. In the 1990s, almost all studies about PRP focused on developing the machine as well as an automated system to separate it for therapeutic use Gonshor, 2002Zimmermann et al., 2001. From the year 2000 to date, there have been several studies related to activating PRP with different agents. In some early studies, PRP was activated by bovine thrombin and Calcium chloride. However, in 2004, Canada and some countries banned the usages of bovine thrombin. After that, some different sources of thrombin were used as replacements, and recombinant human thrombin was considered as the best choice. However, the high price of recombinant human thrombin increased the fee for producing PRP.
PRP application in regenerative medicine
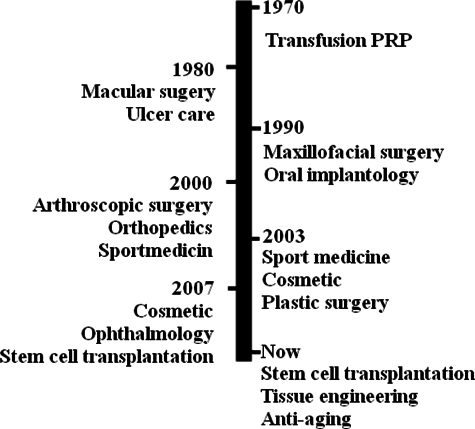
In the 1980s, the definition of regenerative medicine suggested that a patient’s care is related to the use of the patient’s own resources and at the present times, platelets are considered vehicles for the delivery of a balanced pool of healing factors. Some clinical applications in which PRP was used to treat and repair injured tissues and vessels in cutaneous ulcers have been carried out since the 1980s Margolis et al., 2001. Later in the 1990s, platelets were introduced into maxillofacial surgery as fibrin glues. The clinical potential of PRP-therapies has truly achieved positive clinical results in enhanced bone formation and anti-inflammatory functions during oral and maxillofacial applications since that time Anitua, 1999Whitman et al., 1997. To date, PRP has been used in the treatment of more than 30 diseases that belong to facial rejuvenation and plastic surgery, maxillofacial surgery, dentistry and oral surgery, tissue engineering and research, cardiovascular surgery, orthopedic surgery and sports medicine, gastroenterology and urology ( Figure 1 ).
In recent years, PRP is considered in stem cell technology. It is used in both in vitro manipulation and in vivo transplantation. In vitro, PRP is usually supplemented into the cultured medium with some different objects. At first, PRP is used as a replacement of fetal bovine serum in medium. PRP is successfully added into the medium to culture several kinds of mesenchymal stem cells. Pham et al. showed that umbilical cord blood derived mesenchymal stem cells (UCBMSCs) can be primarily cultured by complete medium containing 10% aPRP. UCB-MSCs, isolated using this protocol, maintained their immunophenotype and multilineage differentiation potentials, and did not form tumors when injected at a high dose into athymic nude mice Pham et al., 2014. Human PRP can be seen as an alternative serum source to FCS for MSC cultivation Goedecke et al., 2011. Also, PRP successfully enhances proliferation of human dental stem cells Chen et al., 2012aLee et al., 2011b.
Secondly, PRP is used as a stimulator for stem cell proliferation. We showed that pooled human AB serum and thrombin- activated platelet-rich plasma are alternatives to FCS for AT-MSCs. These human sources are better characterized with regard to potential infectious threats, while providing a higher proliferation rate and retaining differentiation capacity and marker expression of mesenchymal stem cells throughout a long-term culture Goedecke et al., 2011.
Lastly, PRP is a differentiating factor that drives stem cells toward functional cells. PRP induces chondrogenic differentiation of progenitor cells in PGA-HA scaffolds and perhaps, beneficial in scaffold-assisted cartilage repair approaches involving stem and progenitor cells Kruger et al., 2013. PRP, combined with HA scaffolds, trigger bone marrow derived mesenchymal stem cells which may provide additional therapeutic effects on bone regeneration and improve osseointegration in bone defects around dental implants Yun et al., 2013. PRP is a candidate bioactive scaffold capable of releasing endogenous growth factors, and the BMSC and ADSC seeded within the PRP scaffolds differentiate into chondrocytes Van Pham et al., 2013Xie et al., 2012. Umbilical cord blood-derived PRP stimulates dental stem cells to osteogenic cell lineage Lee et al., 2011a. Feng et al. (2010) showed that the co-treatment of PRP and 1,25(OH)(2)D(3) stimulates osteogenic differentiation of adult human mesenchymal stem cells Feng et al., 2010Lin et al., 2006Lu et al., 2008Spero, 1993.
PRP is also used in tissue engineering. In vitro effects of PRP on tissue-engineered cartilages may lead to the creation of engineered cartilage tissues with enhanced properties suitable for cartilage repair Petrera et al., 2013.
Safety profile
Since PRP is prepared from autologous blood, theoretically, there are minimal risks for disease transmission, immunogenic reactions or cancer. Up to date, PRP has been widely used in the treatment of at least ten diseases in more than hundred thousand patients, and there has been no report about the side effects of PRP injection. Wang-Saegusa and colleagues, in their study of over 800 patients, reported no adverse effect following injection of plasma rich in growth factors (PRGF) into the knee joints of these patients for 6 months Wang-Saegusa et al., 2011. Kon and colleagues reported the observation of 91 patients (115 knees) treated with PRP, which showed that PRP treatment is safe, reduces pain and improves knee function, especially in younger patients in 12 months Kon et al., 2011.
Some adverse effects such as infection, injury to nerves or blood vessels were recorded. However, these effects are rare and they depend on the PRP preparation procedure. There was a report about scar tissue formation as well as calcification at the injection site after PRP injection Sampson et al., 2008. Rarely, patients develop some antibodies against clotting factors V and IX Ortel et al., 2001Spero, 1993. However, these effects are only created when PRP is used in systemic injection. To date, there is no evidence of any effect of a local PRP injection. In general, PRP is safe for clinical application. There has not been any critical effect recorded in more than 100,000 cases injected with PRP up till now.
Future of platelet rich plasma in regenerative medicine
Together with stem cells, PRP has become a centre of regenerative medicine in recent years. As a natural cocktail of growth factors, PRP continues to draw the attention of scientists and physicians. Based on the present direction of PRP application, PRP will be widely applied in almost all wounds as well as chronic diseases in combination with stem cells. Both local injection and transfusion of PRP will develop with many new clinical indications. Above all, PRP will become an important adjuvant in stem cell transplantation.
Although PRP is used in the treatment of some diseases, there are still some limitations, which need to be checked in the future for the effectiveness of PRP application in regenerative medicine. At the present, the procedure of PRP preparation is different in some areas of medicine. Also, the quality of PRP has been observed to be clearly different in some areas, such that some authors concluded that PRP is not efficient in wound healing. In the future, a standard operating procedure in PRP preparation is essential. Commercial PRP preparation systems like Biomet GPSIII (Biomet Inc, Warshaw, USA), Arthrex ACP Double syringe system (Arthrex Inc, Naples, USA), RegenPRP (Regen Lab, Switzerland) etc., are yet to become popular. This is probably due to the licensing requirements and the cost involved in the use of such systems. The cost of PRP preparation with these systems is between USD 500 and 1000.
Some concerns should also be addressed, including the effective mechanisms of PRP in wound healing and stem cell proliferation as well as differentiation. Some studies showed that PRP causes differentiation of adipose derived stem cells into chondrocytes Van Pham et al., 2013 while other studies showed that PRP stimulates adipose derived stem cells into osteoblasts Chen et al., 2012b. This difference is related to the difference in PRP procedure preparation, as there is a disparity in component and concentration of growth factors in PRP products. In fact, many studies need to be performed to evaluate the effects of PRP protocols on stem cell proliferation and differentiation. Actually, Perut et al. (2013) showed that the biological activities of platelet concentrates differ according to preparation techniques, which affect platelet and leukocyte content and growth factor availability. Growth factor levels are not always optimal, and can create effects on defective bone healing efficiency Perut et al., 2013. This result is also similar to a previous study carried out by Cho et al., 2011 who showed that varying GF concentrations may result in different biologic effects.
Once these studies are performed with clear results, application of PRP in stem cell transplantation and regenerative medicine will be strongly developed. The artificial PRP should also be suggested for use in the future. Blood collection and PRP protocol are simple and safe; however, blood collection can be objected by patients in some cases, especially in some patients with hematological diseases related to platelet functions.
Conclusion
PRP is a natural biological product containing a high concentration of growth factors. It is a useful tool in regenerative medicine. It can be used as a drug in wound treatment, as an adjuvant in stem cell transplantation, as a differentiating factor in the differentiation of stem cells and as a stimulator in stem cell culture. With these important roles, PRP will continue to be widely used in regenerative medicine in subsequent years. However, more studies about PRP mechanisms related to stem cell differentiation and proliferation need to be carried out to determine the key factors that drive the stem cell proliferation and differentiation. Some concerns about the safety of PRP preparation procedure should also be optimized to eliminate the microorganism contamination.
Abbreviations
ADSC: Adipose derived stem cells; aPRP: Activated platelet rich plasma; BMSC: Bone marrow drived Mesenchymal stem cells; EGF: Epidermal growth factor; FBS: Fetal bovine serum: FCS: Fetal calf serum; FGF: Fibroblast growth factor; IGF: Insulin-like growth factor; PDGF: Platelet-derived growth factor; PRP: Platelet rich plasma; TGF: Transforming growth factor; UCB-MSCs: Umbilical cord blood derived mesenchymal stem cells; VEGF: Vascular endothelial growth factor.
Authors' contributions
All authors read and approved the final manuscript. GRH wrote the Introduction, Healing Process and Platelet Role. TB wrote the Platelet rich plasma and regenerative medicine, Future of Platelet rich plasma, and Conclusion.
References
-
E.
Anitua.
Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. The International journal of oral & maxillofacial implants.
1999;
14
:
529-535
.
-
E.
Anitua,
I.
Andia,
B.
Ardanza,
P.
Nurden,
A.T.
Nurden.
Autologous platelets as a source of proteins for healing and tissue regeneration. Thrombosis and haemostasis.
2004;
91
:
4-15
.
-
E.
Anitua,
M.
Sanchez,
M.M.
Zalduendo,
M.
de la Fuente,
R.
Prado,
G.
Orive,
I.
Andia.
Fibroblastic response to treatment with different preparations rich in growth factors. Cell proliferation.
2009;
42
:
162-170
.
-
S.R.
Browning,
A.M.
Weiser,
N.
Woolf,
S.R.
Golish,
T.P.
SanGiovanni,
G.J.
Scuderi,
C.
Carballo,
L.S.
Hanna.
Platelet-rich plasma increases matrix metalloproteinases in cultures of human synovial fibroblasts. The Journal of bone and joint surgery American volume.
2012;
94
:
e1721-1727
.
-
B.
Carofino,
D.M.
Chowaniec,
M.B.
McCarthy,
J.P.
Bradley,
S.
Delaronde,
K.
Beitzel,
M.P.
Cote,
R.A.
Arciero,
A.D.
Mazzocca.
Corticosteroids and local anesthetics decrease positive effects of platelet-rich plasma: an in vitro study on human tendon cells. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association.
2012;
28
:
711-719
.
-
B.
Chen,
H.H.
Sun,
H.G.
Wang,
H.
Kong,
F.M.
Chen,
Q.
Yu.
The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials.
2012a;
33
:
5023-5035
.
-
L.
Chen,
X.
Lu,
S.
Li,
Q.
Sun,
W.
Li,
D.
Song.
Sustained delivery of BMP-2 and platelet-rich plasma-released growth factors contributes to osteogenesis of human adiposederived stem cells. Orthopedics.
2012b;
35
:
e1402-1409
.
-
H.S.
Cho,
I.H.
Song,
S.Y.
Park,
M.C.
Sung,
M.W.
Ahn,
K.E.
Song.
Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. The Korean journal of laboratory medicine.
2011;
31
:
212-218
.
-
A.
Cieslik-Bielecka,
D.M.
Dohan Ehrenfest,
A.
Lubkowska,
T.
Bielecki.
Microbicidal properties of Leukocyte- and Platelet-Rich Plasma/Fibrin (L-PRP/L-PRF): new perspectives. Journal of biological regulators and homeostatic agents.
2012;
26
:
43S-52S
.
-
J.A.
Coppinger,
G.
Cagney,
S.
Toomey,
T.
Kislinger,
O.
Belton,
J.P.
McRedmond,
D.J.
Cahill,
A.
Emili,
D.J.
Fitzgerald,
P.B.
Maguire.
Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood.
2004;
103
:
2096-2104
.
-
M.
Mos,
A.E.
van der Windt,
H.
Jahr,
H.T.
van Schie,
H.
Weinans,
J.A.
Verhaar,
G.J.
van Osch.
Can platelet-rich plasma enhance tendon repair? A cell culture study. The American journal of sports medicine.
2008;
36
:
1171-1178
.
-
D.M.
Dohan Ehrenfest,
P.
Doglioli,
G.M.
de Peppo,
M.
Del Corso,
J.B.
Charrier.
Choukroun's platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Archives of oral biology.
2010;
55
:
185-194
.
-
L.
Drago,
M.
Bortolin,
C.
Vassena,
S.
Taschieri,
M.
Del Fabbro.
Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC microbiology.
2013;
13
:
4-7
.
-
A.
Drengk,
A.
Zapf,
E.K.
Sturmer,
K.M.
Sturmer,
K.H.
Frosch.
Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells, tissues, organs.
2009;
189
:
317-326
.
-
S.A.
Eming,
T.
Krieg,
J.M.
Davidson.
Inflammation in wound repair: molecular and cellular mechanisms. The Journal of investigative dermatology.
2007;
127
:
514-525
.
-
Y.
Feng,
Y.
Sun,
W.
Jia,
C.
Zhang.
Platelet-rich plasma and 1,25(OH)2 vitamin D3 synergistically stimulate osteogenic differentiation of adult human mesenchymal stem cells. Biotechnology letters.
2010;
32
:
635-642
.
-
V.
Freire,
N.
Andollo,
J.
Etxebarria,
J.A.
Duran,
M.C.
Morales.
In vitro effects of three blood derivatives on human corneal epithelial cells. Investigative ophthalmology & visual science.
2012;
53
:
5571-5578
.
-
O.
Garcia-Martinez,
C.
Reyes-Botella,
L.
Diaz-Rodriguez,
E.
De Luna- Bertos,
J.
Ramos-Torrecillas,
M.F.
Vallecillo-Capilla,
C.
Ruiz.
Effect of platelet-rich plasma on growth and antigenic profile of human osteoblasts and its clinical impact. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons.
2012;
70
:
1558-1564
.
-
A.
Goedecke,
M.
Wobus,
M.
Krech,
N.
Munch,
K.
Richter,
K.
Holig,
M.
Bornhauser.
Differential effect of platelet-rich plasma and fetal calf serum on bone marrow-derived human mesenchymal stromal cells expanded in vitro. Journal of tissue engineering and regenerative medicine.
2011;
5
:
648-654
.
-
A.
Gonshor.
Technique for producing platelet-rich plasma and platelet concentrate: background and process. The International journal of periodontics & restorative dentistry.
2002;
22
:
547-557
.
-
F.
Graziani,
S.
Ivanovski,
S.
Cei,
F.
Ducci,
M.
Tonetti,
M.
Gabriele.
The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clinical oral implants research.
2006;
17
:
212-219
.
-
C.H.
Jo,
J.E.
Kim,
K.S.
Yoon,
S.
Shin.
Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. The American journal of sports medicine.
2012;
40
:
1035-1045
.
-
E.
Kon,
B.
Mandelbaum,
R.
Buda,
G.
Filardo,
M.
Delcogliano,
A.
Timoncini,
P.M.
Fornasari,
S.
Giannini,
M.
Marcacci.
Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association.
2011;
27
:
1490-1501
.
-
B.F.
Kraemer,
R.A.
Campbell,
H.
Schwertz,
M.J.
Cody,
Z.
Franks,
N.D.
Tolley,
W.H.
Kahr,
S.
Lindemann,
P.
Seizer,
C.C.
Yost.
Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS pathogens.
2011;
7
:
e1002355
.
-
J.P.
Kruger,
A.K.
Ketzmar,
M.
Endres,
A.
Pruss,
A.
Siclari,
C.
Kaps.
Human platelet-rich plasma induces chondrogenic differentiation of subchondral progenitor cells in polyglycolic acidhyaluronan scaffolds. Journal of biomedical materials research Part B, Applied biomaterials.
2013
.
-
J.Y.
Lee,
H.
Nam,
Y.J.
Park,
S.J.
Lee,
C.P.
Chung,
S.B.
Han,
G.
Lee.
The effects of platelet-rich plasma derived from human umbilical cord blood on the osteogenic differentiation of human dental stem cells. In vitro cellular & developmental biology Animal.
2011a;
47
:
157-164
.
-
U.L.
Lee,
S.H.
Jeon,
J.Y.
Park,
P.H.
Choung.
Effect of platelet-rich plasma on dental stem cells derived from human impacted third molars. Regenerative medicine.
2011b;
6
:
67-79
.
-
S.S.
Lin,
R.
Landesberg,
H.S.
Chin,
J.
Lin,
S.B.
Eisig,
H.H.
Lu.
Controlled release of PRP-derived growth factors promotes osteogenic differentiation of human mesenchymal stem cells. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference.
2006;
1
:
4358-4361
.
-
H.H.
Lu,
J.M.
Vo,
H.S.
Chin,
J.
Lin,
M.
Cozin,
R.
Tsay,
S.
Eisig,
R.
Landesberg.
Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. Journal of biomedical materials research Part A.
2008;
86
:
1128-1136
.
-
D.
M..
Academy of Sciences, Paris: M. Donne on the Blood Globules. Provincial medical & surgical journal.
1842;
3
:
498-499
.
-
D.J.
Margolis,
J.
Kantor,
J.
Santanna,
B.L.
Strom,
J.A.
Berlin.
Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes care.
2001;
24
:
483-488
.
-
R.E.
Marx.
Platelet-rich plasma (PRP): what is PRP and what is not PRP?. Implant dentistry.
2001;
10
:
225-228
.
-
R.E.
Marx.
Platelet-rich plasma: evidence to support its use. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons.
2004;
62
:
489-496
.
-
A.D.
Mazzocca,
M.B.
McCarthy,
D.M.
Chowaniec,
E.M.
Dugdale,
D.
Hansen,
M.P.
Cote,
J.P.
Bradley,
A.A.
Romeo,
R.A.
Arciero,
K.
Beitzel.
The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. The American journal of sports medicine.
2012;
40
:
1742-1749
.
-
A.
Mishra,
P.
Tummala,
A.
King,
B.
Lee,
M.
Kraus,
V.
Tse,
C.R.
Jacobs.
Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue engineering Part.
2009;
C
:
Methods 15, 431-435
.
-
A.T.
Nurden.
Platelets, inflammation and tissue regeneration. Thrombosis and haemostasis 105 Suppl.
2011;
1
:
S13-33
.
-
T.L.
Ortel,
M.C.
Mercer,
E.H.
Thames,
K.D.
Moore,
J.H.
Lawson.
Immunologic impact and clinical outcomes after surgical exposure to bovine thrombin. Annals of surgery.
2001;
233
:
88-96
.
-
F.
Perut,
G.
Filardo,
E.
Mariani,
A.
Cenacchi,
L.
Pratelli,
V.
Devescovi,
E.
Kon,
M.
Marcacci,
A.
Facchini,
N.
Baldini.
Preparation method and growth factor content of platelet concentrate influence the osteogenic differentiation of bone marrow stromal cells. Cytotherapy.
2013;
15
:
830-839
.
-
M.
Petrera,
J.N.
De Croos,
J.
Iu,
M.
Hurtig,
R.A.
Kandel,
J.S.
Theodoropoulos.
Supplementation with platelet-rich plasma improves the in vitro formation of tissue-engineered cartilage with enhanced mechanical properties. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association.
2013;
29
:
1685-1692
.
-
P.V.
Pham,
N.B.
Vu,
V.M.
Pham,
N.H.
Truong,
T.L.
Pham,
L.T.
Dang,
T.T.
Nguyen,
A.N.
Bui,
N.K.
Phan.
Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. Journal of translational medicine.
2014;
12
:
5-6
.
-
S.
Sampson,
M.
Gerhardt,
B.
Mandelbaum.
Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Current reviews in musculoskeletal medicine.
2008;
1
:
165-174
.
-
J.A.
Spero.
Bovine thrombin-induced inhibitor of factor V and bleeding risk in postoperative neurosurgical patients. Report of three cases. Journal of neurosurgery.
1993;
78
:
817-820
.
-
G.M.
Buul,
W.L.
Koevoet,
N.
Kops,
P.K.
Bos,
J.A.
Verhaar,
H.
Weinans,
M.R.
Bernsen,
G.J.
van Osch.
Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. The American journal of sports medicine.
2011;
39
:
2362-2370
.
-
P.
Van Pham,
K.H.
Bui,
D.Q.
Ngo,
N.B.
Vu,
N.H.
Truong,
N.L.
Phan,
D.M.
Le,
T.D.
Duong,
T.D.
Nguyen,
V.T.
Le.
Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem cell research & therapy.
2013;
4
:
9-1
.
-
A.
Wang-Saegusa,
R.
Cugat,
O.
Ares,
R.
Seijas,
X.
Cusco,
M.
Garcia-Balletbo.
Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Archives of orthopaedic and trauma surgery.
2011;
131
:
311-317
.
-
D.H.
Whitman,
R.L.
Berry,
D.M.
Green.
Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons.
1997;
55
:
1294-1299
.
-
X.
Xie,
Y.
Wang,
C.
Zhao,
S.
Guo,
S.
Liu,
W.
Jia,
R.S.
Tuan,
C.
Zhang.
Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials.
2012;
33
:
7008-7018
.
-
J.H.
Yun,
S.H.
Han,
S.H.
Choi,
M.H.
Lee,
S.J.
Lee,
S.U.
Song,
N.
Oh.
Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: Preliminary study in canine three-wall intrabony defects. Journal of biomedical materials research Part B, Applied biomaterials.
2013
.
-
R.
Zimmermann,
R.
Jakubietz,
M.
Jakubietz,
E.
Strasser,
A.
Schlegel,
J.
Wiltfang,
R.
Eckstein.
Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion.
2001;
41
:
1217-1224
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 01 (2014)
Page No.: 25-31
Published on: 2014-03-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 6033 times
- Download PDF downloaded - 1230 times
- View Article downloaded - 4 times
 Biomedpress
Biomedpress