Abstract
Background: Immunoglobulin G (IgG) is a major immunoglobulin (Ig) in blood that accumulates to a greater extent in the bloodstream of patients impacted by neuroimmunological disorders such as multiple sclerosis (MS). The aim of this study was to determine the effect of IgG obtained from MS patients on the amidolytic activity of coagulation and on anticoagulation factors, and to compare those effects to the effects of IgG from healthy donors.
Methods: Spectrophotometric hydrolysis of specific chromogenic substrate by key haemostasis factors was examined.
Results: Our study shows that unlike healthy individuals, patients suffering from MS express IgG which enhances the amidolytic activity of thrombin and protein C, but inhibits the activity of factor Xa.
Conclusion: Our study shows that IgG and coagulation factors, indeed, interact with each other. IgG may be key mediators of neuroinflammation and, therefore, may serve as a potential target for therapeutic strategies for MS and other neuroimmunological diseases.
Introduction
Multiple sclerosis (MS) is an autoimmune disorder of the central nervous system mediated by different molecular and cellular immune components, which lead to disseminated inflammatory lesions within the brain parenchyma and potential brain damage Bhat and Steinman, 2009Göbel et al., 2016a. The pathogenesis of MS has long been imparted to self-reactive T cells though B cells have also recently been found to play an important role in the development of MS Disanto et al., 2012Sospedra and Martin, 2005. Furthermore, it has been demonstrated that innate immunity plays a pivotal role in the initial pathogenesis as well as in advanced stages of MS Gandhi et al., 2010Mayo et al., 2012Weiner, 2008.
Recent studies suggest that factors of the coagulation cascade traditionally described as an entirely separate entity of the immune system might also be involved in MS development Delvaeye and Conway, 2009. Moreover, several extensive studies have demonstrated the association of disorders of hemostasis cascades and MS. Recent data point to a role of both the extrinsic and the intrinsic coagulation systems.
One factor that has been described within chronic active MS plaques is tissue factor, a glycoprotein considered to be the initiator of the extrinsic coagulation cascade leading to the activation of factor X directly or indirectly through activation of factor IX Han et al., 2008. Activation of factor X mediates the cleavage of prothrombin to thrombin that is able to cleave fibrinogen to fibrin. Interestingly, both fibrinogen deposition and thrombin activation have been reported in human MS lesions or in animal models Adams et al., 2007Davalos et al., 2014Gverić et al., 2003. Furthermore, degradation products of fibrinogen and fibrin (e.g. fibrinopeptide A and D-dimer) have been shown to be significantly upregulated in individuals suffering from MS, while fibrinogen levels were found to be unaltered Aksungar et al., 2008Ehling et al., 2011Liguori et al., 2014. These alterations were also significantly increased in blood samples of MS patients compared to healthy controls Göbel et al., 2016b.
Overall, data from the literature suggest that inhibition of components from both the intrinsic and extrinsic coagulation systems can protect against inflammatory neurodegeneration. For instance, multiple findings support the prominent role of the coagulation system in the development of MS Aksungar et al., 2008Han et al., 2008. Nevertheless, to date, the mechanisms of regulation of coagulation factors in blood of individuals suffering from neuroinflammatory disorders (especially MS) have not been evaluated in detail.
Materials - Methods
Blood plasma samples were taken from 35 healthy donors and 20 patients with MS. Patients were hospitalized in the Neurological Department of Hospital №4 (Kyiv, Ukraine). All donors and patients (or their respective relatives) were informed about the clinical research protocol. Informed consent was obtained in accordance with the Declaration of Helsinki. The clinical research protocol was approved by the Ethics Committees of the ESC (Institute of Biology and Medicine) of Kyiv, Ukraine. Fasting blood samples were collected from the cubital vein of all patients on the first day of hospitalization. Blood was collected into 3.8% sodium citrate solution (at a ratio of 9:1).
IgG was separated by affinity chromatography on protein A sepharose. One ml of blood plasma was applied to the column of protein A Sepharose (total volume of the column was 5 ml). Non-specific bound proteins were washed with 50 mM Tris-HCl buffer containing 130 mM NaCl, pH 7.4. Elution was performed using 0.1 M glycine-HCl buffer, pH 2.2. The purity of separated IgG fractions was controlled by 7.5% PAGE using the following protein standards: myosin (200 kDa), b-galactosidase (116 kDa), phosphorylase b (97 kDa), albumin (66.2 kDa) and ovalbumin (45 kDa). Gels were stained with 0.125% solution of Coomassie Brilliant Blue G-250 in 25% isopropanol and 10% acetic acid. The concentration of the separated IgG was measured by spectrophotometer (Bio-Rad, Hercules, CA).
In order to investigate the influence of IgG on hemostasis in vitro experiments were conducted using a standard set of reagents; “Renam” Russia reagents were used according to the manufacturer’s instructions. IgG obtained from patients with MS as well as from healthy donors were applied to a mixture in two concentrations: 100 and 300 μg/ml.
To examine the influence of IgG on key hemostasis enzymes (thrombin and Factor Xa) in vitro experiments were performed. The following mixture was prepared as follows: 25 μl of enzyme was mixed with 50 mM Tris-HCl containing 130 mM NaCl, pH 7.4 and then IgG was added. After 5 min incubation at 37°C, the corresponding specific chromogenic substrate (in a final concentration of 0.3 mM) was added to the mixture ( Table 1 ).
To examine the influence of IgG on hemostasis enzymes (prothrombin and proenzyme of Protein C) during their zymogen activation the following mixture was prepared as follows: 25μl of healthy donor plasma was mixed with 50 mM Tris-HCl containing 130 mM NaCl, pH 7.4, then with 25 μl of corresponding activators for plasma zymogens, and finally IgG was added ( Table 1 ). After 5min incubation at 37°C, the corresponding specific chromogenic substrate (in a final concentration of 0.3 mM) was added to the mixture ( Table 1 ).
Absorption was measured in two-wave mode at the primary 405 and reference 492 nm wavelengths in a microplate spectrophotometer (QuantTM, BioTek
Instruments, Inc) for 60 minutes. The activity of the evaluated process was proportional to color intensity following release of p-nitroaniline from the chromogenic substrate. The control sample contained the same components but with an equal volume of 50 mM Tris-HCl buffer containing 130 mM NaCl, pH 7.4, instead of IgG.
Statistical analysis of the experimental results was performed in the Origin program. Mean (M) and standard deviation (SD) were calculated for each group. A statistically significant difference was set at P<0.05. Statistical analysis of electrophoregrams was performed in the TotalLab 2. 01 program.
Results
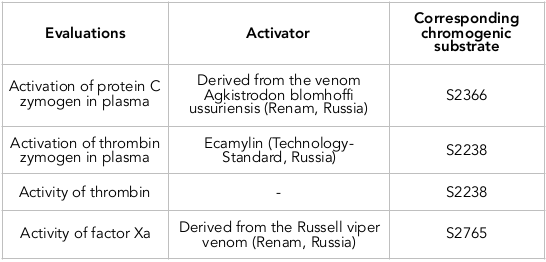
In this study, IgG from MS patients was shown to have the ability to affect the amidolytic activity of hemostasis enzymes such as thrombin, factor X and protein C. The effect of IgG from blood plasma of the MS patients was significantly greater compared to the effect of IgG from healthy donors.
Thrombin is a protease in blood that facilitates blood clotting by converting fibrinogen to fibrin. There was an increase of amidolytic activity of thrombin under influence of fractions of IgG in both concentration of 100 and 300 μg/ml ( Figure 1 ). In normal conditions without IgG in mixture, the level of amidolytic thrombin activity was equal to 0.485±0.013 relative units (r.u.). After applying IgG from healthy donors to the mixture, the level of thrombin activity after 60 minutes of incubation was 45% higher (for the 100 μg/ml IgG concentration) and 35% higher (for the 300 μg/ml IgG concentration). However, IgG obtained from blood plasma of patients with MS induced a stronger effect, after application of IgG from MS patients. Thus, IgG from MS patients, at a concentration of 100 μg/ml, increased thrombin activity by 30%; at a concentration of 300 μg/ml, IgG from MS patients increased the activity by 58% ( Figure 1 ). In comparison to the effect of IgG from healthy donors on thrombin activity, IgG from MS patients (at the same concentration of 300 μg/ml) was 17% greater.
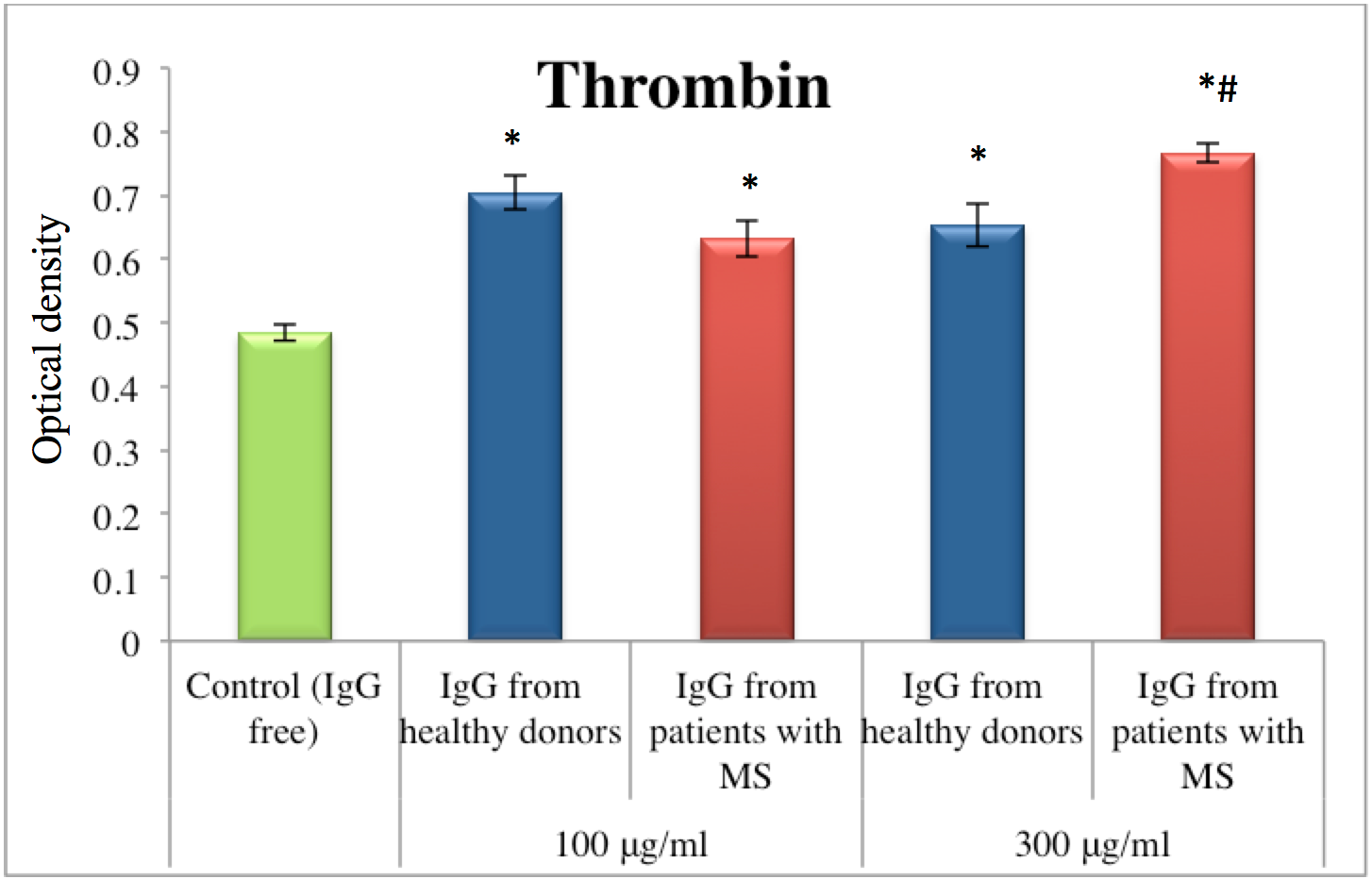
The opposite trend was observed for factor Xa (also known as the eponym Stuart–Prower factor). Unlike IgG from healthy donors, IgG from MS patients inhibited amidolytic activity of factor Xa. For example, IgG from MS patients (at a concentration of 100 μg/ml) inhibited factor Xa activity by 23%. At a concentration of 300 μg/ml, IgG from MS patients inhibited factor Xa activity by 5% ( Figure 2 ). Overall, compared to the effect of IgG from healthy donors, the inhibitory effect of IgG from MS patients was 28% greater (at the 100 μg/ml IgG concentration) and 14% greater (at the 300 μg/ml IgG concentration).
Next, the amidolytic activity of hemostasis enzymes was measured after activation of the corresponding zymogens in blood plasma. Activation was achieved by addition of the specific endogenic activators in medium ( Table 1 ). This technique can help address questions about specificity of the tested reactions. The effect of IgG in the blood plasma remains unclear. To address this, we applied the IgG from healthy donors and the corresponding activators instead of key enzymes in the medium during incubation.
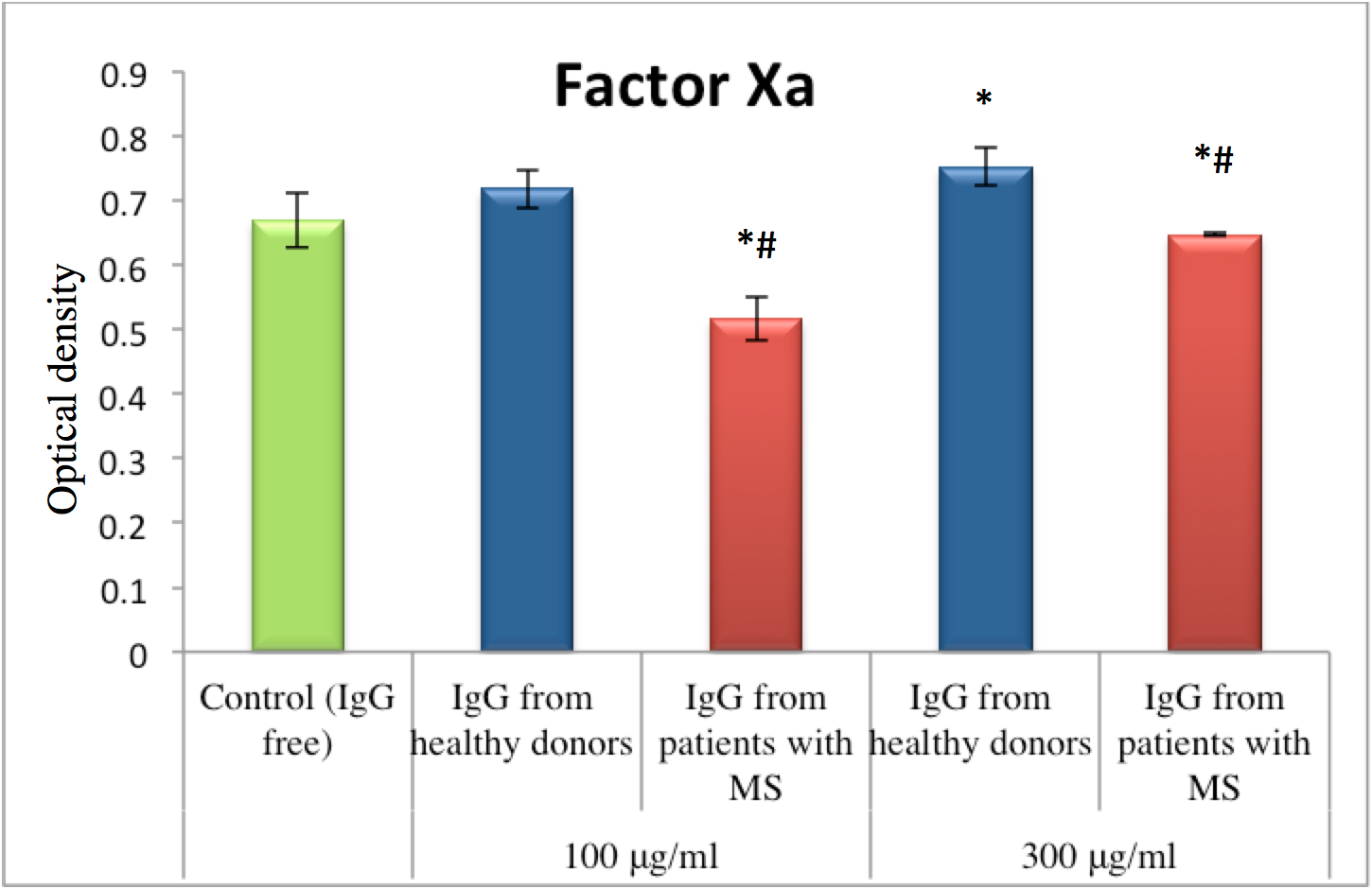
The results showed an effect of IgG on thrombin followed by prothrombin activation in plasma under the influence of activator derived from the venom Echis multisquamatus (ecamylin). Thus, IgG obtained from MS patients as well as IgG from healthy donors both showed activation of amidolytic activity of thrombin after its activation of prothrombin in plasma. In the control probe without IgG, the level of amidolytic thrombin activity was equal to 0.458±0.021 r.u. After applying healthy donor IgG to the mixture, the level of thrombin activity after 60 minutes of incubation was 42% (for the 100 μg/ml IgG concentration) and 22% (for the 300 μg/ml IgG concentration) greater compared to the control. Meanwhile, the level of thrombin activity after applying IgG from MS patients was 49% (for the 100 μg/ml IgG concentration) and 32% (for the 300 μg/ml IgG concentration) greater compared to the control ( Figure 3 ).
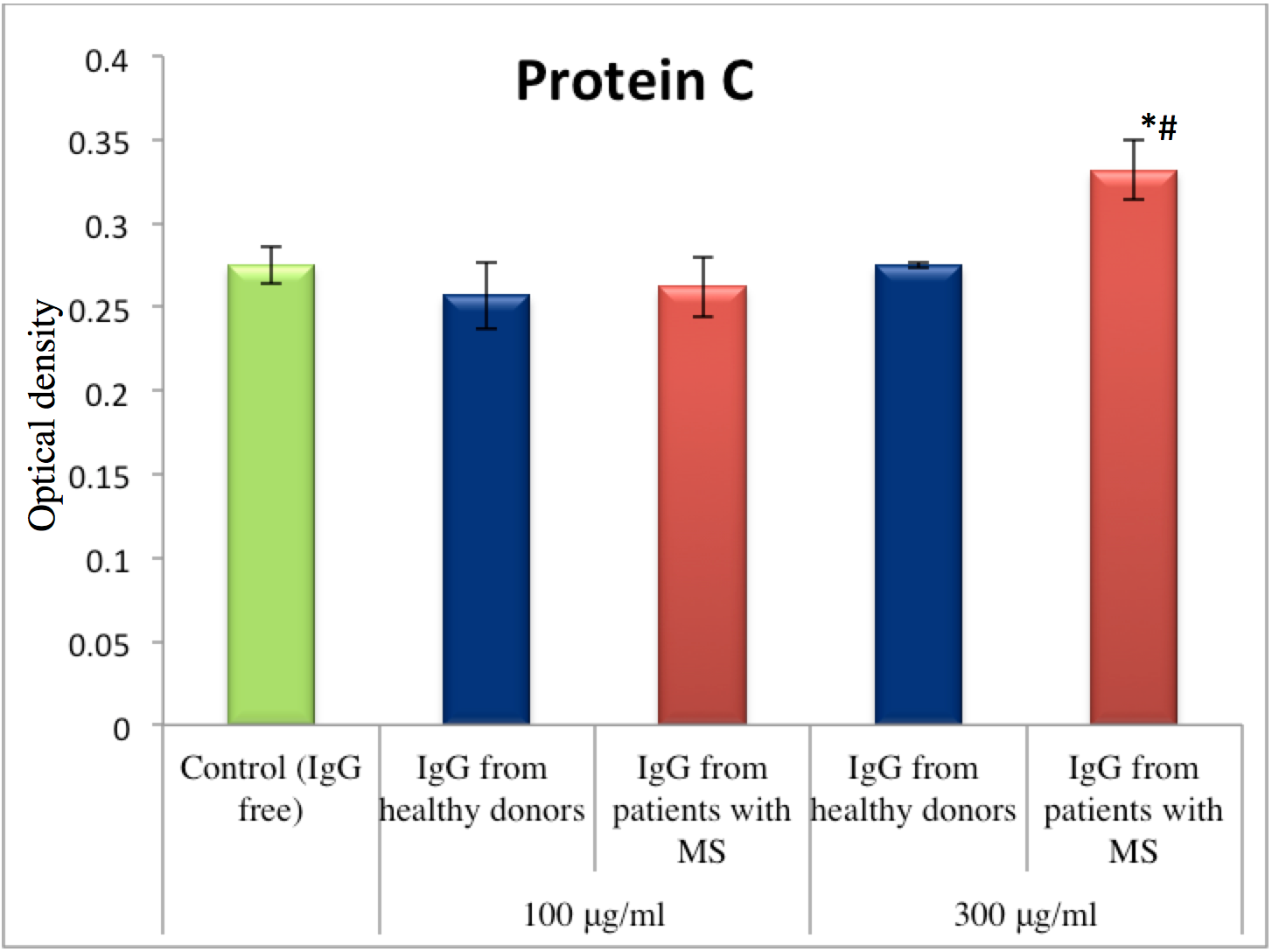
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIV, plays an important role in regulating anticoagulation, inflammation and cell death, and in maintaining the permeability of blood vessel walls. The amidolytic activity of Protein C was measured in plasma after activation of its zymogen under the influence of activator derived from the venom Agkistrodon blomhoffi ussuriensis. A statistically significant difference was observed under the influence of MS-derived IgG at a concentration of 300 μg/ml. The level of Protein C activation was elevated by 21% compared to the control ( Figure 4 ).
Discussion
In this study, we showed that the amidolytic activity of coagulation as well as anticoagulation factors were altered under the influence of IgG obtained from patients with MS (a prototypic neuroinflammatory disease). As comparison, the effect of IgG obtained from healthy donors was also evaluated. For the most part, an increase of amidolytic activity for both thrombin and protein C were observed. However, an inhibition of factor Xa activity was also observed. According to the literature, prothrombin and factor Xa are strongly elevated in individuals suffering from MS Bhat and Steinman, 2009. Prothrombin and other hemostasis factors have been described to potentially enhance inflammation in artherosclerotic plaques, sepsis, endotoxemia and encephalomyelitis Borissoff et al., 2011Schoenmakers et al., 2005Strukova, 2001. We showed an effect of IgG on the activity of coagulation as well as anticoagulation factors.
Although the role of hemostasis enzymes in the interactions with IgG need to be further studied, the described coagulation factors may represent key mediators in neuroinflammation Bhat and Steinman, 2009Göbel et al., 2016b. Accordingly, the interactions between IgG and coagulation factors may provide new targets for the development of future therapeutic strategies for MS and possibly other neuroimmunological diseases. Moreover, they may serve as possible biomarkers for disease monitoring.
Conclusion
In this study, we demonstrated that IgG is able to exert an effect on the enzymes of hemostasis system. There was an observed elevation of amidolytic activity of thrombin, as well as thrombin and protein C activated from their zymogens in blood plasma, after addition of IgG from MS patients (at a concentration of 300 μg/ml). The influence of MS-derived IgG fractions (at 100 and 300 μg/ml concentrations) on thrombin, in a system without plasma or after its activation in plasma, provide evidence of a potentially direct impact of IgG on thrombin. These revelations may influence future therapeutic strategies for MS. Moreover, inhibition of amidolytic activity of factor Xa was also observed. However, correlation between the level of inhibition and IgG concentration was absent. IgG obtained from healthy donors also affected the tested reactions in some measure too. A mechanism of concurrent inhibition might be occurring, but future investigations are needed to evaluate that.
Abbreviation
IgG: Immunoglobulin G
MS: multiple sclerosis
Author Contribution
All authors contributed in manuscript preparation. Katrii T.B., Shandyuk V.Yu. obtained data and analyzed it. Vovk T.B., Halenova T.I., Raksha N.G. interpreted of data analysis. Katrii T.B. designed of figures. Shershnov O.V., Melnyk V.S, Savchuk O.M., Ostapchenko L.I. performed designed the study. All authors drafted the first version and approve the final draft.
References
-
R.A.
Adams,
J.
Bauer,
M.J.
Flick,
S.L.
Sikorski,
T.
Nuriel,
H.
Lassmann,
J.L.
Degen,
K.
Akassoglou.
The fibrin-derived γ377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. Journal of Experimental Medicine.
2007;
204
:
571-582
.
-
F.B.
Aksungar,
A.E.
Topkaya,
Z.
Yildiz,
S.
Sahin,
U.
Turk.
Coagulation status and biochemical and inflammatory markers in multiple sclerosis. Journal of Clinical Neuroscience.
2008;
15
:
393-397
.
-
R.
Bhat,
L.
Steinman.
Innate and adaptive autoimmunity directed to the central nervous system. Neuron.
2009;
64
:
123-132
.
-
J.I.
Borissoff,
H.M.
Spronk,
H.
ten Cate.
The hemostatic system as a modulator of atherosclerosis. New England Journal of Medicine.
2011;
364
:
1746-1760
.
-
D.
Davalos,
K.M.
Baeten,
M.A.
Whitney,
E.S.
Mullins,
B.
Friedman,
E.S.
Olson,
J.K.
Ryu,
D.S.
Smirnoff,
M.A.
Petersen,
C.
Bedard.
Early detection of thrombin activity in neuroinflammatory disease. Annals of neurology.
2014;
75
:
303-308
.
-
M.
Delvaeye,
E.M.
Conway.
Coagulation and innate immune responses: can we view them separately?. Blood.
2009;
114
:
2367-2374
.
-
G.
Disanto,
J.
Morahan,
M.
Barnett,
G.
Giovannoni,
S.
Ramagopalan.
The evidence for a role of B cells in multiple sclerosis. Neurology.
2012;
78
:
823-832
.
-
R.
Ehling,
F.
Di Pauli,
P.
Lackner,
B.
Kuenz,
W.
Santner,
A.
Lutterotti,
C.
Gneiss,
H.
Hegen,
M.
Schocke,
F.
Deisenhammer.
Fibrinogen is not elevated in the cerebrospinal fluid of patients with multiple sclerosis. Fluids and Barriers of the CNS.
2011;
8
:
25
.
-
R.
Gandhi,
A.
Laroni,
H.L.
Weiner.
Role of the innate immune system in the pathogenesis of multiple sclerosis. Journal of neuroimmunology.
2010;
221
:
7-14
.
-
K.
Göbel,
P.
Kraft,
S.
Pankratz,
C.C.
Gross,
C.
Korsukewitz,
R.
Kwiecien,
R.
Mesters,
B.E.
Kehrel,
H.
Wiendl,
C.
Kleinschnitz.
Prothrombin and factor X are elevated in multiple sclerosis patients. Annals of neurology.
2016a;
80
:
946-951
.
-
K.
Göbel,
S.
Pankratz,
C.-M.
Asaridou,
A.M.
Herrmann,
S.
Bittner,
M.
Merker,
T.
Ruck,
S.
Glumm,
F.
Langhauser,
P.
Kraft.
Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nature communications.
2016b;
7
:
11626
.
-
D.
Gverić,
B.
Herrera,
A.
Petzold,
D.A.
Lawrence,
M.L.
Cuzner.
Impaired fibrinolysis in multiple sclerosis: a role for tissue plasminogen activator inhibitors. Brain.
2003;
126
:
1590-1598
.
-
M.H.
Han,
S.-I.
Hwang,
D.B.
Roy,
D.H.
Lundgren,
J.V.
Price,
S.S.
Ousman,
G.H.
Fernald,
B.
Gerlitz,
W.H.
Robinson,
S.E.
Baranzini.
Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature.
2008;
451
:
1076
.
-
M.
Liguori,
A.
Qualtieri,
C.
Tortorella,
V.
Direnzo,
A.
Bagala,
M.
Mastrapasqua,
P.
Spadafora,
M.
Trojano.
Proteomic profiling in multiple sclerosis clinical courses reveals potential biomarkers of neurodegeneration. PloS one.
2014;
9
:
e103984
.
-
L.
Mayo,
F.J.
Quintana,
H.L.
Weiner.
The innate immune system in demyelinating disease. Immunological reviews.
2012;
248
:
170-187
.
-
S.H.
Schoenmakers,
P.H.
Reitsma,
C.A.
Spek.
Blood coagulation factors as inflammatory mediators. Blood.
2005;
Cells
:
Molecules, and Diseases 34, 30-37
.
-
M.
Sospedra,
R.
Martin.
Immunology of multiple sclerosis. Annu Rev Immunol.
2005;
23
:
683-747
.
-
S.
Strukova.
Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry (Moscow).
2001;
66
:
8-18
.
-
H.L.
Weiner.
A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. Journal of neurology.
2008;
255
:
3-11
.
Comments
Downloads
Article Details
Volume & Issue : Vol 4 No 08 (2017)
Page No.: 1502-1512
Published on: 2017-08-16
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3551 times
- Download PDF downloaded - 1255 times
- View Article downloaded - 14 times
 Biomedpress
Biomedpress