Cytokine induced killer cell immunotherapy in cancer treatment: from bench to bed side
Abstract
Cytokine-induced killer (CIK) cells are T effector cells generated by monocytes cultured and stimulated by cytokines. CIK cells were studied for more than 20 years ago. Because they can cause lysis of tumor cells that of both autologous and allogenic origins, they were used in cancer treatment. This review aimed to summarize advancements of CIK cell research and their recent clinical applications in cancer treatment. In general, CIK cells were widely clinically used for recent 5 years. They gave promising results in breast cancer, lung cancer, renal cancer, and hepatocellular carcinoma treatment. Looking into the future, CIK cell based immunotherapy will become an important tool in cancer treatment.
Introduction
Adoptive cell therapy of cancer demonstrated in mice more than 50 years ago Mitchison, 1955. During 50 years, some adoptive cell therapies were developed with many bright results in both pre-clinic and clinic. To date, more than 10.000 patients with cancer were treated by adoptive cell therapies. Most of them used CIK cell therapy and dendritic cell (DC) therapy. Different to DCs that cause tumor antigenspecific immune responses, CIK cell therapy can create nonantigen specific immune responses. CIK cells possess non- MHC-restricted cytolytic activities against to cancer cells. From some particular mechanisms of cancer cell recognition, CIK cells can detect and attack cancer cells. This review summarizes some characteristics of CIK cells and an update of clinical applications of CIK cells in cancer treatment.
Cik cell phenotypes
CIK cells are a heterogeneous population of T lymphocytes with NK phenotypes and functional properties. This cell population was named “cytokine induced killer” cells because they were generated under effects of cytokines. These CIK cells can cause toxic malignant cells followed the manner of MHC-unrestricted cytotoxicity. CIK cells were produced by incubation of peripheral blood monocytes in medium plus with INF-gamma, mAb anti-CD3 and IL-2 Schmidt-Wolf et al., 1991. After 2-3 weeks of culture, CIK cells are expanded in vitro from few to more than 1000 fold Marin et al., 2006Pals et al., 2007Thorne et al., 2006.
As heterogeneous population, CIK cells contain at least 3 cell sub-populations included CD3+CD56+, CD3+CD56-, CD3- CD56+ with largest population CD3+CD56+. Some studies determined anti-tumor activity in CIK cells belongs to fraction CD3+CD56+ Edinger et al., 2003Pals et al., 2007. In fact, in the human body, this population always exists with phenotype of lymphocytes accounting from 1-5% of T cells Maccalli et al., 2007. With this phenotype, CIK cells hold both lymphocytes and natural killer cells activities.
In vitro, CIK cells easily were produced by culture of peripheral blood mononuclear cells in medium plus with INFgamma on day 0, and anti-CD3 OKT3 (50 ng/ml) and IL-2 (500 IU/mL) on the next day, followed by the addition of IL-2 during the culture. INF-gamma was used to increase the cytotoxicity of CIK cells, while anti-CD3 acted as a mitogenic agent on T cells. The cocktail with INF-gamma, anti-CD3 and IL-2 holds an important role that triggers the T cell expansion and increases the T cell cytotoxicity. So that by the in vitro cell culture, CIK cells were expanded with a large amount and high activity of T cells and NK cells. Some studies showed that CIK cells produced IL-2, IL-7 and IL-12. So that to produce CIK cells, besides IL-2 addition, some authors used IL-7 and IL-12 also could produce high antitumor activity CIK cells Farag et al., 2002Huang et al.,2006. To date, almost studies used CIK cells were induced from peripheral blood, but CIK cells also can be produced from bone marrow and cord blood Alvarnas et al.,2001Introna et al., 2006.
Mechanism of anti-tumor activity
The mechanism of anti-tumor activity of CIK cells has not completely clarified. However, some pathways related to this activity were identified. It is showed that CIK cells significantly inhibited the anti-tumor activity when they were blocked lymphocyte function associated antigen -1 (LFA-1) and cell adhesion molecule -1 (ICAM-1) Schmidt-Wolf et al., 1996Schmidt-Wolf et al., 1993. In fact, CIK cells were treated with dibutyryl (db)-cAMP that prevents the conversion of LFA-11 into a high affinity receptor for ICAM-1, were inhibited to release perforin and granzyme Mehta et al.,1995.
Another mechanism also was detected about tumor recognition of CIK cells that similar in NK cells. CIK cells recognize the tumor antigen via NKG2D. NKG2D (Natural killer group 2 member D) is one member of the c-type lectinactivating receptor family. NKG2D is expressed on all NK cells. Ligands for NKG2D were restrictedly expressed in malignant cells Diefenbach et al., 2000Jamieson et al., 2002. When induced with IFN-gamma, IL-2 and anti-CD3, NKG2D was highly expressed in CIK cells. Interaction between NKG2D and tumor antigen that expressed in tumor cells will cause the cytolytic effect by perforin and granzyme release of CIK cells.
Besides the recognition of CIK cells by NKG2D, CIK cells also specifically attack tumor cells by another mechanism. Recent studies showed that co-culture of CIK cells and induced DCs that can present tumor antigen can enhance the CIK cells to attack tumor cells Marten et al., 2001Nagaraj et al., 2004Ziske et al., 2001.
Clinical trials
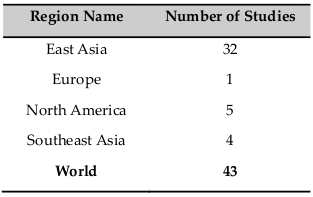
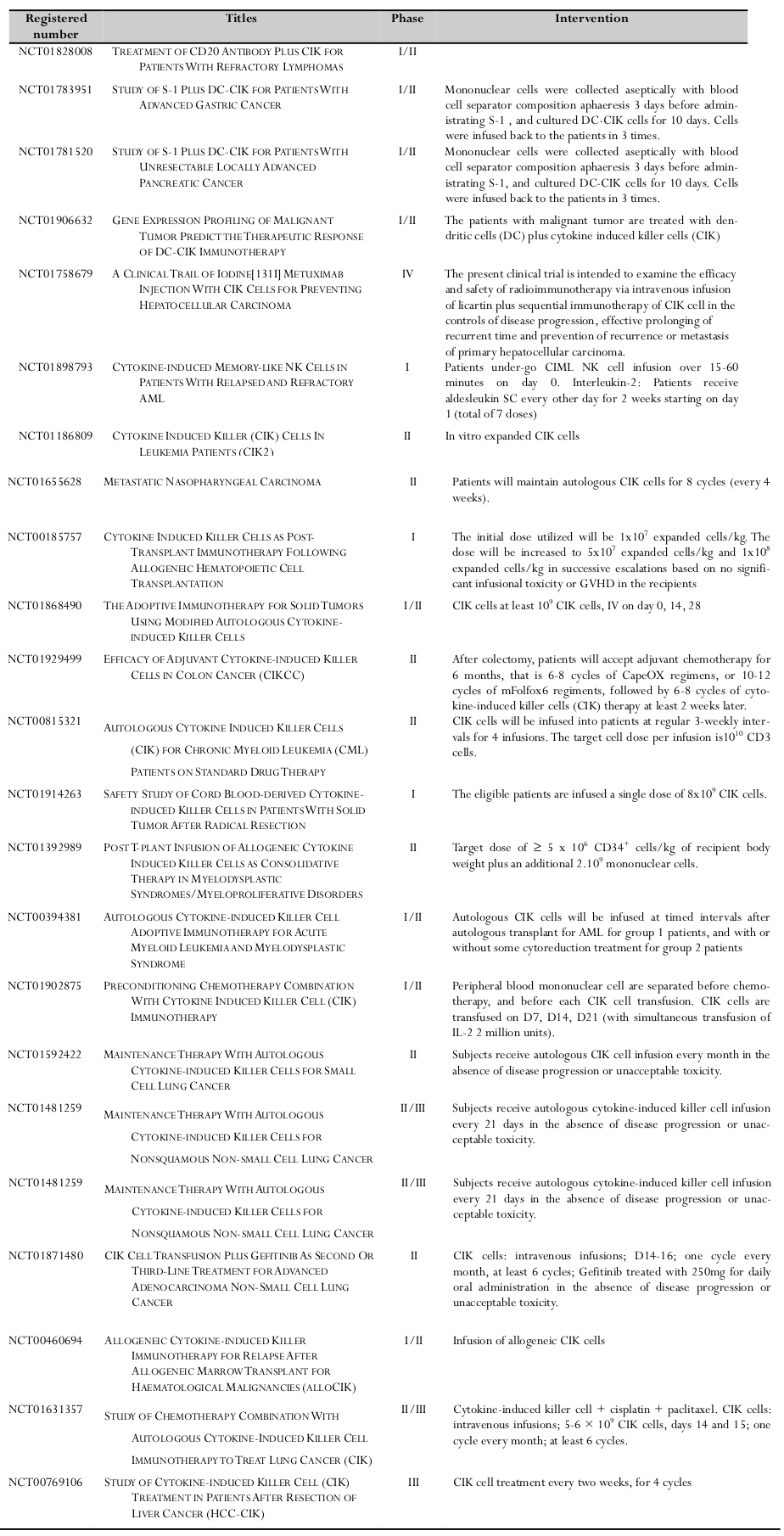
CIK cells were used in clinical trials for some diseases ( Table 1 , Table 2 ). Particularly, CIK cell therapy in combination with some conventional therapies or dendritic cells was studied in Phase III of lung cancer, in Phase IV of hepatocellular carcinoma. Moreover, CIK cell therapy also used in clinical trials for other cancers such as ovarian cancer, colorectal cancer, renal cancer, leukemia, breast cancer…
In HCC, to increase the efficacy of trans-catheter arterial chemoembolization (TACE) on HCC treatment, Hao et al. (2010) combined TACE and CIK cells. The results showed that the efficacy of TACE was significantly improved. Wang et al. (2013) showed that the disease control rate was 67.7% in patients transfused with CIK cells Wang et al.,2013. Similarly, in HCC patients, Huang et al. (2013) showed that CIK cell immunotherapy was valuable therapeutic strategy to prevent recurrence and metastasis in HCC patients after TACE and RFA Huang et al., 2013. Effects of CIK cell transfusion caused a significant increased of CD3+, CD4+, CD4+CD8+ and CD3+CD4+ T cells Ma et al., 2012, improved the immunological status in HCC patients Shi et al., 2004, reduce the level of serum AFP and anti-HBV and decrease the 1-year recurrence rate of patients with HCC after curative TACE plus RFA Pan et al., 2010.
CIK cells represent a promising immunotherapy for the treatment of gastrointestinal tumors Jakel et al., 2014. Adjuvant transfusion of CIK cells prolongs DFS in patients with colorectal cancer from 29.35 ± 6.39 % in control without CIK cells to 59.65 ± 24.80 % with CIK transfusion, and no immediate adverse reactions to the CIK cell transfusions Zhu et al., 2013. DC/CIK therapy also reduced the risk of postoperative disease progression with an increased OS in gastric and colorectal cancer patients Gao et al., 2014.
DC-CIK cell therapy markedly prolongs survival time enhances immune function and improves the efficacy of the treatment of breast cancer patients. In treated patients, the percentage of T cells (CD3+, CD4+ and CD4+CD8+), CD16+ monocytes, and CD3+CD56+ natural killer T cells, the levels of interleukin-2, interleukin-12, tumor necrosis factor-α, interferon-γ, and nucleolar organizer region protein in the peripheral blood of cancer patients was significantly increased Wang et al., 2014b. DC-CIK was feasible and effective in treating advanced renal cancer Kim et al., 2014Su et al.,2010. Wang et al. showed an objective response rate (ORR) of 39% and a disease control rate (DCR) of as 75%. No clinically significant side effects were observed Wang et al., 2014a. In another study, Zhan et al. combined CIK cells with DCs and showed that TL-pulsed DC-CIK cells could prevent recurrence/metastasis and increase the overall survival rate after surgery in localized or locally advanced renal carcinoma with the overall survival rates significantly higher in the DC-CIK group and IFN-α group than that in the control group Zhan et al., 2012.
CIK cells also were successfully used in ovarian cancer treatment. Liu at al. (2014) transfused CIK cells in a clinical with 94 patients with IIB-IV grade ovarian cancer. They showed that a median PFS were 37.7 months in the treatment group and 22.2 months in the control group. After 2 courses of CIK cells transfusion, the proportion of CD4CD25CD127 regular T cells in the peripheral blood significantly decreased Liu et al., 2014.
In lung cancer, CIK cells have important effects on antitumor in both early-stage disease (I-IIIA) Li et al., 2012 and advanced stage disease (IIIB-V) Li et al., 2012Shi et al., 2012Zhong et al., 2011. By the research with 87 paired patients, Li et al. (2012) suggested that CIK cell immunotherapy could improve the efficacy of conventional chemotherapy in NSCLC patients, and increased frequency of CIK cell treatment could further enhance the beneficial effects. Yang et al. (2013) also confirmed that CIK cells plus DC could increased the 1- and 2-year overall survival rates up to 57.2 compared to 27.0 % in control Yang et al., 2013.
Recent studies used CIK cells to target cancer stem cells (CSCs). Gammaitoni et al (2013) demonstrated that CIK tumor killing activity against melanoma CSCs was intense and comparable with results reported against differentiated metastatic melanoma cells. CIK cell transplantation resulted in delayed tumor growth, increased necrotic areas, and lymphocyte infiltration at tumor sites Gammaitoni et al., 2013.
Conclusion
Immune therapies are promising therapies for cancer treatment. CIK cells hold high anti-tumor activity in MHC unrestricted manner. These cells are easily produced with a large amount from peripheral blood by cultured peripheral blood mononuclear cells with INF-gamma, IL-2 and anti-CD3. CIK cells have used to treat several cancers with good results. Especially, CIK cells based therapy clinically studied in phase IV in hepatocellular carcinoma, and phase III in lung cancer. From these results, it hopes that CIK cell therapy rapidly pushes into routine application in lung cancer or hepatocellular carcinoma in the near future.
Abbreviations
CIK: Cytokine-induced killer; DC: Dendritic cell; HCC: Hepatocellular carcinoma; ICAM-1: Cell adhesion molecule - 1; LFA-1: Lymphocyte function associated antigen-1; MHC: Major histocompatibility complex; NK: Natural killer cells; NKG2D: Natural killer group 2 member D; TACE: Transcatheter arterial chemoembolization
References
-
J.C.
Alvarnas,
Y.C.
Linn,
E.G.
Hope,
R.S.
Negrin.
Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation.
2001;
7
:
216-222
.
-
A.
Diefenbach,
A.M.
Jamieson,
S.D.
Liu,
N.
Shastri,
D.H.
Raulet.
Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature immunology.
2000;
1
:
119-126
.
-
M.
Edinger,
Y.A.
Cao,
M.R.
Verneris,
M.H.
Bachmann,
C.H.
Contag,
R.S.
Negrin.
Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood.
2003;
101
:
640-648
.
-
S.S.
Farag,
T.A.
Fehniger,
L.
Ruggeri,
A.
Velardi,
M.A.
Caligiuri.
Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood.
2002;
100
:
1935-1947
.
-
L.
Gammaitoni,
L.
Giraudo,
V.
Leuci,
M.
Todorovic,
G.
Mesiano,
F.
Picciotto,
A.
Pisacane,
A.
Zaccagna,
M.G.
Volpe,
S.
Gallo.
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clinical cancer research : an official journal of the American Association for Cancer Research.
2013;
19
:
4347-4358
.
-
D.
Gao,
C.
Li,
X.
Xie,
P.
Zhao,
X.
Wei,
W.
Sun,
H.C.
Liu,
A.T.
Alexandrou,
J.
Jones,
R.
Zhao.
Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PloS one.
2014;
9
:
e9388-6
.
-
J.
Huang,
K.W.
Kerstann,
M.
Ahmadzadeh,
Y.F.
Li,
M.
El-Gamil,
S.A.
Rosenberg,
P.F.
Robbins.
Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. Journal of immunology.
2006;
(Baltimore
:
Md : 1950) 176, 7726-7735
.
-
Z.M.
Huang,
W.
Li,
S.
Li,
F.
Gao,
Q.M.
Zhou,
F.M.
Wu,
N.
He,
C.C.
Pan,
J.C.
Xia,
P.H.
Wu.
Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. Journal of immunotherapy.
2013;
(Hagerstown
:
Md : 1997) 36, 287-293
.
-
M.
Introna,
M.
Franceschetti,
A.
Ciocca,
G.
Borleri,
E.
Conti,
J.
Golay,
A.
Rambaldi.
Rapid and massive expansion of cord blood-derived cytokine-induced killer cells: an innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone marrow transplantation.
2006;
38
:
621-627
.
-
C.E.
Jakel,
A.
Vogt,
M.A.
Gonzalez-Carmona,
I.G.
Schmidt-Wolf.
Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. Journal of immunology research.
2014;
2014
:
89721-4
.
-
A.M.
Jamieson,
A.
Diefenbach,
C.W.
McMahon,
N.
Xiong,
J.R.
Carlyle,
D.H.
Raulet.
The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity.
2002;
17
:
19-29
.
-
J.S.
Kim,
I.S.
Chung,
S.H.
Lim,
Y.
Park,
M.J.
Park,
J.Y.
Kim,
Y.G.
Kim,
J.T.
Hong,
Y.
Kim,
S.B.
Han.
Preclinical and clinical studies on cytokine-induced killer cells for the treatment of renal cell carcinoma. Archives of pharmacal research.
2014;
37
:
559-566
.
-
R.
Li,
C.
Wang,
L.
Liu,
C.
Du,
S.
Cao,
J.
Yu,
S.E.
Wang,
X.
Hao,
X.
Ren,
H.
Li.
Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer.
2012;
immunology
:
immunotherapy : CII 61, 2125-2133
.
-
J.
Liu,
H.
Li,
S.
Cao,
X.
Zhang,
J.
Yu,
J.
Qi,
X.
An,
W.
Yu,
X.
Ren,
X.
Hao.
Maintenance therapy with autologous cytokineinduced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. Journal of immunotherapy.
2014;
(Hagerstown
:
Md : 1997) 37, 115-122
.
-
Y.
Ma,
Y.C.
Xu,
L.
Tang,
Z.
Zhang,
J.
Wang,
H.X.
Wang.
Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Experimental hematology & amp; oncology.
2012;
1
:
1-1
.
-
C.
Maccalli,
D.
Nonaka,
A.
Piris,
D.
Pende,
L.
Rivoltini,
C.
Castelli,
G.
Parmiani.
NKG2D-mediated antitumor activity by tumor-infiltrating lymphocytes and antigen-specific T-cell clones isolated from melanoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research.
2007;
13
:
7459-7468
.
-
V.
Marin,
E.
Dander,
E.
Biagi,
M.
Introna,
G.
Fazio,
A.
Biondi,
G.
D'Amico.
Characterization of in vitro migratory properties of anti-CD19 chimeric receptor-redirected CIK cells for their potential use in B-ALL immunotherapy. Experimental hematology.
2006;
34
:
1219-1229
.
-
A.
Marten,
C.
Ziske,
B.
Schottker,
S.
Renoth,
S.
Weineck,
P.
Buttgereit,
F.
Schakowski,
A.
von Rucker,
T.
Sauerbruch,
I.G.
Schmidt-Wolf.
Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations. Journal of immunotherapy.
2001;
(Hagerstown
:
Md : 1997) 24, 502-510
.
-
B.A.
Mehta,
I.G.
Schmidt-Wolf,
I.L.
Weissman,
R.S.
Negrin.
Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood.
1995;
86
:
3493-3499
.
-
N.A.
Mitchison.
Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. The Journal of experimental medicine.
1955;
102
:
157-177
.
-
S.
Nagaraj,
C.
Ziske,
I.G.
Schmidt-Wolf.
Human cytokine-induced killer cells have enhanced in vitro cytolytic activity via non-viral interleukin-2 gene transfer. Genetic vaccines and therapy.
2004;
2
:
1-2
.
-
S.T.
Pals,
D.J.
de Gorter,
M.
Spaargaren.
Lymphoma dissemination: the other face of lymphocyte homing. Blood.
2007;
110
:
3102-3111
.
-
C.C.
Pan,
Z.L.
Huang,
W.
Li,
M.
Zhao,
Q.M.
Zhou,
J.C.
Xia,
P.H.
Wu.
Serum alpha-fetoprotein measurement in predicting clinical outcome related to autologous cytokine-induced killer cells in patients with hepatocellular carcinoma undergone minimally invasive therapy. Chinese journal of cancer.
2010;
29
:
596-602
.
-
I.G.
Schmidt-Wolf,
P.
Lefterova,
V.
Johnston,
C.
Scheffold,
M.
Csipai,
B.A.
Mehta,
T.
Tsuruo,
D.
Huhn,
R.S.
Negrin.
Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cellular immunology.
1996;
169
:
85-90
.
-
I.G.
Schmidt-Wolf,
P.
Lefterova,
B.A.
Mehta,
L.P.
Fernandez,
D.
Huhn,
K.G.
Blume,
I.L.
Weissman,
R.S.
Negrin.
Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Experimental hematology.
1993;
21
:
1673-1679
.
-
I.G.
Schmidt-Wolf,
R.S.
Negrin,
H.P.
Kiem,
K.G.
Blume,
I.L.
Weissman.
Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. The Journal of experimental medicine.
1991;
174
:
139-149
.
-
M.
Shi,
B.
Zhang,
Z.R.
Tang,
Z.Y.
Lei,
H.F.
Wang,
Y.Y.
Feng,
Z.P.
Fan,
D.P.
Xu,
F.S.
Wang.
Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World journal of gastroenterology : WJG.
2004;
10
:
1146-1151
.
-
S.B.
Shi,
T.H.
Ma,
C.H.
Li,
X.Y.
Tang.
Effect of maintenance therapy with dendritic cells: cytokine-induced killer cells in patients with advanced non-small cell lung cancer. Tumori.
2012;
98
:
314-319
.
-
X.
Su,
L.
Zhang,
L.
Jin,
J.
Ye,
Z.
Guan,
R.
Chen,
T.
Guo.
Immunotherapy with cytokine-induced killer cells in metastatic renal cell carcinoma. Cancer biotherapy & amp; radiopharmaceuticals.
2010;
25
:
465-470
.
-
S.H.
Thorne,
R.S.
Negrin,
C.H.
Contag.
Synergistic antitumor effects of immune cell-viral biotherapy. Science (New.
2006;
York
:
NY) 311, 1780-1784
.
-
D.
Wang,
B.
Zhang,
H.
Gao,
G.
Ding,
Q.
Wu,
J.
Zhang,
L.
Liao,
H.
Chen.
Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC cancer.
2014a;
14
:
25-1
.
-
X.P.
Wang,
M.
Xu,
H.F.
Gao,
J.F.
Zhao,
K.C.
Xu.
Intraperitoneal perfusion of cytokine-induced killer cells with local hyperthermia for advanced hepatocellular carcinoma. World journal of gastroenterology : WJG.
2013;
19
:
2956-2962
.
-
Z.X.
Wang,
J.X.
Cao,
M.
Wang,
D.
Li,
Y.X.
Cui,
X.Y.
Zhang,
J.L.
Liu,
J.L.
Li.
Adoptive cellular immunotherapy for the treatment of patients with breast cancer: A meta-analysis. Cytotherapy.
2014b
.
-
L.
Yang,
B.
Ren,
H.
Li,
J.
Yu,
S.
Cao,
X.
Hao,
X.
Ren.
Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer.
2013;
immunology
:
immunotherapy : CII 62, 65-73
.
-
H.L.
Zhan,
X.
Gao,
X.Y.
Pu,
W.
Li,
Z.J.
Li,
X.F.
Zhou,
J.G.
Qiu.
A randomized controlled trial of postoperative tumor lysate-pulsed dendritic cells and cytokine-induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma. Chinese medical journal.
2012;
125
:
3771-3777
.
-
R.
Zhong,
J.
Teng,
B.
Han,
H.
Zhong.
Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer.
2011;
immunology
:
immunotherapy : CII 60, 1497-1502
.
-
Y.
Zhu,
H.
Zhang,
Y.
Li,
J.
Bai,
L.
Liu,
Y.
Liu,
Y.
Qu,
X.
Qu.
Efficacy of postoperative adjuvant transfusion of cytokineinduced killer cells combined with chemotherapy in patients with colorectal cancer. Cancer.
2013;
immunology
:
immunotherapy : CII 62, 1629-1635
.
-
C.
Ziske,
A.
Marten,
B.
Schottker,
P.
Buttgereit,
F.
Schakowski,
M.
Gorschluter,
A.
von Rucker,
C.
Scheffold,
N.
Chao,
T.
Sauerbruch.
Resistance of pancreatic carcinoma cells is reversed by coculturing NK-like T cells with dendritic cells pulsed with tumor-derived RNA and CA 19-9. Molecular therapy : the journal of the American Society of Gene Therapy.
2001;
3
:
54-60
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 02 (2014)
Page No.: 71-77
Published on: 2014-05-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3600 times
- Download PDF downloaded - 950 times
- View Article downloaded - 6 times
 Biomedpress
Biomedpress