Micronucleus Assessment as a Biomarker and Susceptibility to DNA Damage in Workers Occupationally Exposed to Pesticides
Abstract
Occupational exposures to hazardous chemicals are common in industries using solvent based materials as well as in indoor environments where people are exposed to volatile organic compounds from various sources. The health of the workers has several determinants, including risk factors at the workplace leading to cancer. The aim of the present investigation was to assess the potential cytogenetic damage associated with occupational exposure among pesticide workers by using micronuclei and other nuclear abnormalities as a biomarker. The micronucleus assay on exfoliated buccal cells is a useful and minimally invasive method for monitoring genetic damage in humans. To determine the genotoxic effects of pesticide workers, Micronucleus assay was carried out in exfoliated buccal cells of 50 pesticide workers and 50 controls. For each individual, 2,000 exfoliated buccal cells were analyzed. Micronucleus and other nuclear abnormalities frequencies in exposed were significantly higher than those in control groups (P < 0.05) and also significantly related to smoking, tobacco chewing and alcohol drinking habit (P < 0.05). Increased frequency of these nuclear abnormalities in buccal epithelial cells of exposed workers indicates adverse cellular reaction and/or a surveillance mechanism to eliminate cells with genetic damage. The present studied individuals may be at a higher risk of developing cancer and therefore monitored for any long term adverse effects of the exposure. Genotoxic studies are foremost for any occupational exposure studies. Evaluation based on genotoxic parameters is often useful in warranting environmental endowment and occupational health. Genotoxicity biomarkers have received a considerable interest as tools for detecting human genotoxic exposure and effects, especially in health surveillance programs dealing with chemical carcinogens.
Introduction
To live in the 21st century means to live in a toxic world, where we are exposed daily to numerous environmental toxins and pollutants. Occupational health deals with all aspects of health and safety in the workplace and has a strong focus on primary prevention of hazards. The health of the workers has several determinants, including risk factors at the workplace leading to cancers, accidents, musculoskeletal diseases, respiratory diseases, hearing loss, circulatory diseases, stress related disorders and communicable diseases etc. Humans are diverse in their responses to exogenous exposures because of variability, the rate of metabolism, DNA repair processes and other factors Anwar, 1997. Occupational exposures to hazardous chemicals are common in industries using solvent based materials as well as in indoor environments where people are exposed to volatile organic compounds from various sources Barroa et al., 2009.
Health suffers the influence of inherited, nutritional, and environmental factors. Populations of industrial areas are intensely exposed to chemical substances that can cause mutations, cancer, and congenital defects Hirvonen, 1995. Pesticides, as a heterogeneous category of biologically active compounds, are characterized by various degrees of toxicity also to non-target species, including human beings. Most pesticides are acutely toxic to humans. The widespread use of pesticides in agriculture, public health and household environments results in continuous exposure of human populations. With green revolution and industrialization, they have become household items of the agriculturists. Unfortunately, because of their easy availability and accessibility, they have also been commonly abused for suicidal purpose in the developing countries. According to World Health Organization, three million cases of acute poisoning occur annually, of which about 2,20,000 die. Of these, 99% of the fatal poisonings occur in developing countries, particularly among farm workers (WHO, 1962). Exposure to low-level of pesticides is known to produce a variety of biochemical changes, some of which may be responsible for the adverse biological effects reported in human and experimental studies Gupta et al., 1998Banerjee et al., 1999. Conversely, some biochemical alterations may not necessarily lead to clinically recognizable symptoms, although all the biochemical responses can be used as markers of exposure or effect. The biochemical changes induced after exposure to pesticides or their active metabolites include target cell/receptor binding, protein and DNA adduct formation, and induction or inhibition of enzymes Heinzow and McLean,1994.
One way to study the effects on an exposed population is to conduct monitoring studies, using pertinent biological parameters with a short term manifestation, such as cytogenetic analysis, by which damages to the DNA or to the chromosomes resulting from exposure can be identified. Micronucleus (MN) assay for exfoliated cells have been used to evaluate the genotoxic effects produced by low doses of carcinogenic substances or carcinogenic mixtures, to which human populations are exposed Maluf and Erdtmann, 2000. Micronuclei originated from aberrant mitosis and consist of acentric chromosomes, chromatid fragments or whole chromosomes that have failed to be incorporated in to the daughter nuclei during mitosis. The micronuclei test is the most frequent technique used to detect chromosome breakage or mitotic interference events thought to be associated with increased risk for cancer Fairbain et al., 1995.
Knowledge of human health risks related to environmental exposure to hazardous chemical agents is a current concern Franco et al., 2008. To add further knowledge to the genetic risk on an exposed population, we applied the MN and other nuclear abnormalities (NA) as a biomarker. Our objective was to evaluate the cytogenetic damage in exfoliated buccal cells obtained from pesticide workers and non-exposed control subjects, further to establish the relationship of the MN frequency with occupational and non-occupational factors, such as the smoking, tobacco chewing and drinking habits.
Materials - methods
Study Subjects
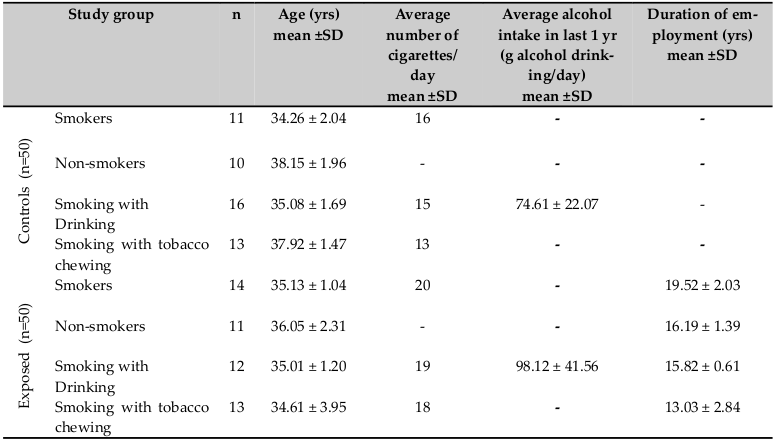
The study population composed of 50 exposed pesticide workers and 50 unexposed controls. The exposed group included 14 smokers and 11 non-smokers, 12 smokers with alcohol drinking and 13 smokers with tobacco chewing habit, from 8 pesticide industries located in the rural area of Coimbatore City, South India. The respective control groups were matched for age and sex (11 smokers and 10 nonsmokers, 16 smokers with alcohol drinking and 13 smokers with tobacco chewing habit) and had no occupational exposition to toxic agents. At the time of sample collection the subjects signed a term of informed consent. All subjects were selected based on questionnaire which included items about age, occupational exposure, smoking habit, use of drugs, such as alcohol, virus illnesses, recent vaccinations, and radiological exams. All the individuals who agreed to participate in the study were healthy, and they answered a detailed questionnaire according to the protocol published by the International Commission for Protection against Environmental Mutagens and Carcinogens Carrano, 1988. For the exposed group, a further questionnaire was completed to evaluate the use of protective measure. These workers were engaged in work for more than 8 h per day with a minimum 7 yrs of exposure duration. None of these study groups showed significant differences with regard to lifestyle and personal factors. The study procedures used in the present study were approved by the Institutional ethical committee.
Cell sampling
Before sampling, each subject rinsed the mouth thoroughly with tap water. Buccal cells (BCs) were collected from consented volunteers at the end of the work shift according to the criteria established by Tolbert and his co-workers Tolbert et al., 1991. Prior to BC collection the mouth was rinsed thoroughly with water to remove any unwanted debris. Buccal cell samples were obtained by rubbing the inside of both cheeks using a wooden spatula. The cells were collected in tubes containing 3 mL sterile saline.
Micronucleus analysis
Ten micro liters of buccal mucosal cell suspension was smeared on a microscopic slide, dried in air and fixed with cold methanol: acetic acid (3:1) solution in 0.1M phosphate buffer (pH 7.5) for 20 min. Then the slides were stained by Feulgen reaction essentially by the modified procedure of Belien and co-workers Belien et al., 1995. In briefly, hydrolysis in 5N HCl for 10 min at room temperature, washing in distilled water for 5 min followed by staining with fresh Schiff’s reagent (Sigma Chem, USA) for 90 min, and washing in tap water for 15 min. The cells were counter stained in Coplin jars containing 1% Fast Green reagent for 2-5 min and rinsed with distilled water. Slides were analyzed under light microscope (Leitz, Germany) with 1000 x magnification. A total of minimum 2000 cells per individual were scored for analysis of micronuclei. The slides were randomized and scored by a single observer. Micronuclei were scored in normal cells. In addition, the frequencies of nuclear anomalies, namely binucleates, broken eggs, karyolysis were recorded. MN and other nuclear abnormalities were classified according to Tolbert and co-workers. MN must satisfy the following conditions: a) consist of nuclear material; b) be completely separated from the parent nucleus; c) be less than 1/3 of the diameter of associated nuclei; d) be smooth, oval- or roundshaped; e) be on the same plane of focus and f) be of the same color, texture and refraction as the main nucleus. Cells with two nuclei were considered to be binucleate. Besides MN, other NA, such as Binucleates (BN), broken eggs’ (nuclei that appeared cinched), karyolysis (dissolution of nucleus) was recorded separately.
Statistical analysis
The samples were coded at the time of preparation and scoring. They were decoded before statistical analysis for comparison. The significance of the differences between control and exposed group means were analyzed using Student’s t-test, whereas Pearson’s rank correlation analyses were performed to assess the association between end-points and the independent variables. The MNC, BNC, BEC and KLC distributions of individuals, grouped by each of two-class factors, were compared with the Mann-Whitney test. All the calculations were performed using SPSS 11.01 statistical software (SPSS Inc., Chicago, IL).
Results
The characteristics of the subjects used in the study are shown in Table 1 . The individuals were classified according to their age, length of occupation, smoking, tobacco chewing, and alcohol drinking habits.
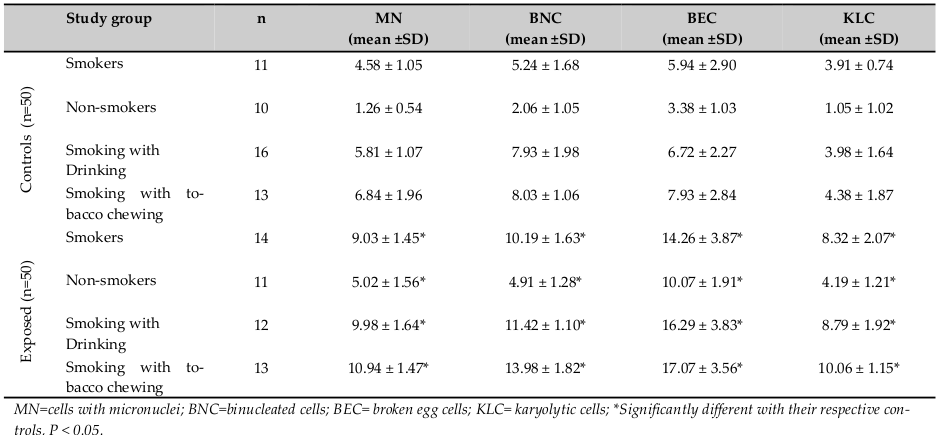
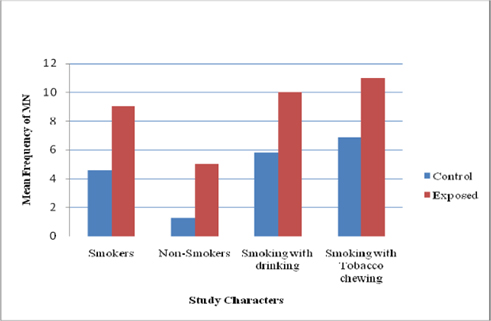
Results on micronuclei frequency and nuclear abnormalities are given in Table 2 . Assessment of MN frequencies in exfoliated buccal cells revealed a significant difference (P < 0.05) between exposed workers with smoking (9.03±1.45) and controls with smoking habit (4.58±1.05). Smoking also had a marked effect on MN frequency among unexposed control group (4.58 vs. 1.26 between smokers and non-smokers, respectively). The average MN frequency in the exposed nonsmokers was 5.02±1.56, and in the control non-smokers was 1.26±0.54 (P < 0.05). The average micronucleus frequencies were 9.98 and 5.81±1.07 between exposed smoking with drinking habit and unexposed, respectively ( Figure 1 ).
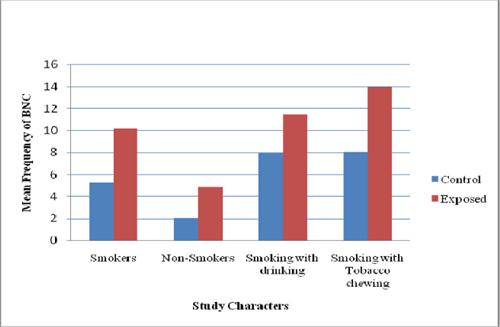
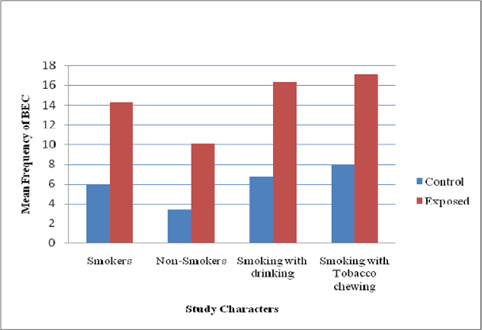
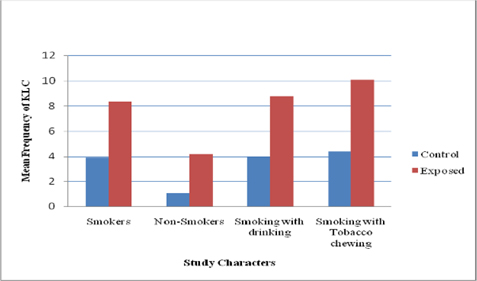
Like MN, A significant difference (P<0.05) in other NA was more prevalent in pesticide workers compared with that of controls ( Figure 2 and Figure 3 ). Among the three NA, karyolysis was predominant in smokers with chewing habit followed by smokers with drinking habit ( Figure 4 ). Burgaz et al., 1995 reported a significant increase of micronucleated cells (P < 0.001) in smokers, as compared to non-smokers. Similarly our study showed synergistic effect between habitual usage and occupational exposure. We observed a significant increase in the frequency of micronuclei formation and nuclear changes in buccal epithelial cells. Individuals of the exposed as well as control groups with smoking habit and chewing habit showed an enhanced frequency of micronuclei in comparison to controls.
Discussion
Exposure to environmental agents and byproducts of cellular metabolism results in DNA damage, which if left unrepaired, could lead to the process of carcinogenesis. The molecular epidemiologic studies have shown that cancer is mediated by both genetic and environmental factors that play a role in the individual’s susceptibility to carcinogenesis. The development of cancer is not only due to endogenous or exogenous carcinogens, but also to their interactions with genes that are also involved in the detoxification of these carcinogens.
The molecular epidemiologic studies have shown that cancer is mediated by both genetic and environmental factors that play a role in the individual’s susceptibility to carcinogenesis. Genetic variation in the ability to repair DNA lesions induced by smoking tobacco or environmental carcinogens may contribute to the inter-individual variation in cancer. Humans are exposed to a large number of physical or chemical agents, which can cause a variety of health hazards. Majority of human cancers are known to arise as a direct consequence of environmental exposure to mutagenic and carcinogenic agents, mainly through diet, habit, and occupation.
Excesses in risk have been found in most Aronson et al., 1996Fleming et al., 1999Alavanja et al., 2003Mills and Yang, 2003Settimi et al., 2003Ritchie et al., 2003, but not all Van Der Gulden and Vogelzang, 1996 studies investigating the relation between exposure to pesticides and prostate cancer. Some other studies have reported excess risk among farmers or herbicide applicators. Yet, farmers perform a wide variety of tasks, and are therefore exposed to numerous potential carcinogenic substances like solvents, fuels and oils, pesticides, and more.
Exposure to pesticides has been associated with an increase in the incidence of non-Hodgkin’s lymphoma Hardell and Eriksson, 1999Zheng et al., 2001, multiple myeloma Khuder and Mutgi, 1997, soft tissue sarcoma Kogevinas et al., 1995, lung sarcoma Blair et al., 1993, pancreatic, stomach, liver, bladder and gall bladder cancer Ji et al., 2001Shukla et al., 2001, Parkison’s disease Gauthier et al., 2001 and reproductive outcomes Arbuckle et al., 2001, among others. Regarding pesticide exposure, many reports dealing with chromosomal aberrations Au et al., 1999Zeljezic et al., 2001, sister chromatid exchange Shaham et al., 2001Zeljezic et al., 2002, micronuclei Falck et al., 1999Pastor et al., 2003 and Comet cells Grover et al.,2003 found significant increases in these biomarkers, providing suggestive evidence of genotoxic effects induced by pesticides. Dittberner et al., 1997 reported alcohol use can increase the number of micronuclei. Piyathilake et al., 1995 and Sarto et al., 1997 have reported smoking also increase the MN frequency in buccal cells.
These results show that individuals with smoking, drinking, tobacco chewing and pesticide exposure to have significantly higher frequencies of MN induction, indicative of cytogenetic damage in these individuals. Our earlier study provides an evidence of MN and other nuclear abnormalities in the buccal mucosa cells of automobile mechanics and spray painters exposed to PAHs RafiqKhan and Sudha, 2012Mohammed RafiqKhan and Sudha, 2013. The use of the micronuclei test (MNT) to detect and quantify the genotoxic action of carcinogenics is well established in several systems, either in vitro or in vivo, its sensitivity being compared to the analysis of chromatid breaks and exchanges Kliesch and Adler, 1980. This test presents great advantages over other techniques, not requiring cell culture nor metaphase preparations, it is applicable on interphase cells, is a good indicator of chromosome mutations, is not invasive and has a low cost Calvert et al., 1998Titenko-Holland et al., 1998. When humans interact with chemicals in their workplaces or natural environments, it may result in both detoxification or activation processes, which ultimately can lead to an interaction with hemoglobin, proteins, DNA, or normal cells and the formation biological end products that may result in genotoxic effects if no biological repair is done. Several factors including the host, agent, and the environment influence how the disease interaction will progress. Our study showed that smoking status affected genetic damage in both groups studied, but a significant association emerged only among exposed workers. In our study, smoking, tobacco chewing, and alcohol drinking is associated with a significant induction of MN and other NA among exposed pesticide workers. This shows synergistic effect between habitual usage and occupational exposure.
Conclusion
Correlations among biomarkers, an important issue in biomarker research, provides enhanced insight and understanding of the complexity encountered on a molecular level during exposure to genotoxic agents. Explorations of correlations between biomarkers will contribute to the development of human monitoring to genotoxic exposures and will help to select optimal biomarkers for more efficient monitoring of various human exposures. MN and other NA, such as binucleates, karyorrhexis and karyolysis are also useful indices of chemical exposure and toxic response. Stages of biomonitoring exist such that within this progression from exposure to disease, systems can be developed to detect any adverse changes.
The present investigation suggests that pesticide workers under their particular conditions of exposure revealed clear evidence of genotoxicity in exfoliated buccal cell when evaluated by MN test. This shows synergistic effect between habitual usage and occupational exposure. Besides elevated MN frequency, exposed individuals also exhibited raised prevalence of other NAs like BNC, BEC and KLC. These abnormalities occur at an elevated level in response to cellular injury. These workers may not be aware that they have been exposed to genotoxic agents nor do they know the type and amount of agent to which they have been exposed. Therefore, there is a need to educate those who work with chemicals, about the potential occupational hazards and the importance of using protective measures. The rate of occupational diseases and injuries are very high in India, and thousands of workers are routinely exposed to various chemicals, there is currently little or no survey data due to lack of proper reporting or monitoring procedures. Nonetheless, it is important to create better awareness of occupational hazards among workers to promote occupational safety. The obtained information can be used as an early warning about the potential risk of health problems’ developing in the long run, also more comprehensive, larger-scale study is needed to further explore the effects of gene-environment interactions.
Abbreviations
BCs: Buccal cells; BEC: Broken egg cells; BNC: Binucleate cells; KLC: Karyolytic cells; MN: Micronucleus; MNT: Micronucleus test; NA: Nuclear abnormalities; PAHs: Polycyclic aromatic hydrocarbons; WHO: World health organization.
Authors’ contributions
All authors read and approved the final manuscript. MRK designed the study, GTK and RK contributed to the study design, literature review and analyzed the data. SNS, UDP and YRR participated in the designing of the study. MRK prepared the manuscript in cooperation with all the other authors.
References
-
M.C.
Alavanja,
C.
Samanic,
M.
Dosemeci,
J.
Lubin,
R.
Tarone,
C.F.
Lynch.
Use of agricultural pesticides and prostate cancer risk in the agricultural health study cohort. Am J Epidemiol.
2003;
157
:
800-814
.
-
W.A.
Anwar.
Biomarkers of human exposure to pesticides. Environ Health Perspect.
1997;
105
:
801-806
.
-
T.E.
Arbuckle,
Z.
Lin,
L.S.
Mery.
An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ Health Perspect.
2001;
109
:
851-857
.
-
K.J.
Aronson,
J.
Siemiatycki,
R.
Dewar,
M.
Gerin.
Occupational risk factors for prostate cancer: results from a case-control study in Montreal, Quebec, Canada. Am J Epidemiol.
1996;
143
:
363-373
.
-
W.W.
Au,
C.H.
Sierra-Torres,
N.
Cajas-Salazar,
B.K.
Shipp,
M.S.
Legator.
Cytogenetic effects from exposure to mixed pesticides and the influence from genetic susceptibility. Environ Health Perspect.
1999;
107
:
501-505
.
-
B.D.
Banerjee,
V.
Seth,
A.
Bhattacharya,
S.T.
Pasha,
A.K.
Chakraborty.
Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett.
1999;
107
:
33-47
.
-
R.
Barroa,
J.
Regueirob,
M.
Llompart,
C.G.
Jaresb.
Analysis of industrial contaminants in indoor air: Part 1. Volatile organic compounds, carbonyl compounds, polycyclic aromatic compounds and polychlorinated biphenyls. J Chromatography.
2009;
1216
:
540-566
.
-
J.A.M.
Belien,
M.P.
Copper,
B.J.M.
Braakhuis,
G.B.
Snow,
J.P.A.
Baak.
Standardization of counting micronuclei: definition of a protocol to measure genotoxic damage in human exfoliated cells. Carcinogenesis.
1995;
16
:
2395-2400
.
-
A.
Blair,
D.J.
Grauman,
J.H.
Lubin,
J.F.
Fraumeni Jr.
Lung cancer and other causes of death among licensed pesticide applicators. J Natl Cancer Inst.
1993;
71
:
31-37
.
-
S.
Burgaz,
A.
Iscan,
Z.K.
Buyukbingol,
A.
Bozkurt,
A.E.
Karakaya.
Evaluation of micronuclei in exfoliated urothelial cells and urinary thioether excretion of smokers. Mutat Res.
1995;
335
:
163-169
.
-
G.M.
Calvert,
G.
Talaska,
C.A.
Mueller.
Genotoxicity in workers exposed to methyl bromide. Mutat Res.
1998;
417
:
115-128
.
-
A.V.
Carrano.
Considerations for population monitoring using cytogenetic techniques. Mutat Res.
1988;
204
:
379-406
.
-
U.
Dittberner,
B.
Schmetzer,
P.
Golzer,
G.
Eisenbrand,
H.
Zankl.
Genotoxic effects of 2-transhexenal in human buccal mucosa cells in vivo. Mutat Res.
1997;
390
:
161-165
.
-
D.W.
Fairbain,
P.L.
Olive,
K.L.
O’Neil.
The Comet assay: A comprehensive review. Mutat Res.
1995;
339
:
37-59
.
-
G.C.
Falck,
A.
Hirvonen,
R.
Scarpato,
S.T.
Saarikoski,
L.
Migliore,
H.
Norppa.
Micronuclei in blood lymphocytes and genetic polymorphism for GSTM1, GSTT1 and NAT2 in pesticide- exposed greenhouse workers. Mutat Res.
1999;
441
:
225-237
.
-
L.E.
Fleming,
J.A.
Bean,
M.
Rudolph,
K.
Hamilton.
Cancer incidence in a cohort of licensed pesticide applicators in Florida. J Occup Environ Med.
1999;
41
:
279-288
.
-
S.S.
Franco,
A.C.
Nardocci,
W.M.
Gunther.
PAH biomarkers for human health risk assessment: A review of the state-of-the-art. Cad Saude Publica.
2008;
24
:
569-580
.
-
E.
Gauthier,
I.
Fortier,
F.
Courchesne,
P.
Pepin,
J.
Mortimer,
D.
Gauvreau.
Environmental pesticide exposure as a risk factor for Alzheimer’s disease: A case-control study. Environ Res.
2003;
86
:
37-45
.
-
P.
Grover,
K.
Danadevi,
M.
Mahboob,
R.
Rozati,
B.S.
Banu,
M.F.
Rahman.
Evaluation of genetic damage in workers employed in pesticide production utilizing the Comet assay. Mutagenesis.
2003;
18
:
201-205
.
-
A.
Gupta,
A.
Gupta,
G.S.
Shukla.
Effects of neonatal quinalphos exposure and subsequent withdrawal on free radical generation and antioxidative defenses in developing rat brain. J Appl Toxicol.
1998;
18
:
71-77
.
-
L.
Hardell,
M.A.
Eriksson.
Case-control study of non-Hodgkin lymphoma and exposure to pesticides. Cancer.
1999;
85
:
1353-1360
.
-
B.G.J.
Heinzow,
A.
McLean.
Critical evaluation of current concepts in exposure assessment. Clin Chem.
1994;
40
:
1368-1375
.
-
A.
Hirvonen.
Genetic factors in individual responses to environmental exposures. J Occupat Environ Med.
1995;
1
:
37-43
.
-
B.T.
Ji,
D.T.
Silverman,
P.A
Stewart,
G.M.
Swanson.
Occupational exposure to pesticides and pancreatic cancer. Am J Ind Med.
2001;
39
:
92-99
.
-
S.A.
Khuder,
A.B.
Mutgi.
Meta-analyses of multiple myeloma and farming. Am J Ind Med.
1997;
32
:
510-516
.
-
U.
Kliesch,
I.D.
Adler.
Sensitivity comparison of chromosome analysis and micronucleus test in mouse bone marrow. Mutat Res.
1980;
74
:
160
.
-
M.
Kogevinas,
T.
Kauppinen,
R.
Winkelmann,
H.
Becher,
P.A.
Bertaz- zi.
Soft tissue sarcoma and non-Hodgkin’s lymphoma in workers exposed to phenoxy herbicides, chlorophenols, and dioxins: Two nested case-control studies. Epidemiology.
1995;
6
:
396-402
.
-
S.W.
Maluf,
B.
Erdtmann.
Evaluation of occupational risk in Brazilian hospital. Genet Mol Biol.
2000;
23
:
485-488
.
-
P.K.
Mills,
R.
Yang.
Prostate cancer risk in California farm workers. J Occup Environ Med.
2003;
45
:
249-258
.
-
Sudha
Mohammed RafiqKhan.
Elevated frequencies of micronuclei and other nuclear abnormalities in buccal epithelial cells of spray painters in South India. Int J Pharm Lifesci.
2013;
4
:
2680-2684
.
-
S.
Pastor,
A.
Creus,
T.
Parron,
A.
Cebulska-Wasilewska,
C.
Siffel,
S.
Piperakis,
R.
Marcos.
Biomonitoring of four European populations occupationally exposed to pesticides: Use of micronuclei as biomarkers. Mutagenesis.
2003;
18
:
249-258
.
-
J.
Piyathilake,
M.
Macaluso,
R.J.
Hine,
D.W.
Vinter,
E.
Richards,
C.L.
Krumdieck.
Cigarette smoking, intracellular vitamin deficiency and occurrence of micronuclei in epithelial cells of the buccal mucosa. Cancer Epidemiol Biomarkers Prev.
1995;
4
:
751-758
.
-
M.
RafiqKhan,
S.
Sudha.
Evaluation of genotoxicity in automobile mechanics occupationally exposed to polycyclic aromatic hydrocarbons using micronuclei and other nuclear abnormalities. Iran J Cancer Prev.
2012;
5
:
87-92
.
-
J.M.
Ritchie,
S.L.
Vial,
L.J.
Fuortes,
L.W.
Robertson,
H.
Guo,
V.E.
Reedy.
Organochlorines and risk of prostate cancer. J Occup Environ Med.
2003;
45
:
692-702
.
-
F.
Sarto,
S.
Finetto,
L.
Giacomelli,
D.
Mazotti,
R.
Tomanin,
A.G.
Levis.
Micronucleus assay in exfoliated cells of human buccal mucosa. Mutagenesis.
1997;
2
:
11-17
.
-
L.
Settimi,
A.
Masina,
A.
Andrion,
O.
Axelson.
Prostate cancer and exposure to pesticides in agricultural settings. Int J Cancer.
2003;
104
:
458-461
.
-
J.
Shaham,
Z.
Kaufman,
R.
Gurvich,
Z.
Levi.
Frequency of sister-chromatid exchange among greenhouse farmers exposed to pesticides. Mutat Res.
2001;
491
:
71-80
.
-
Y.
Shukla,
A.
Arora.
Transplacental carcinogenic potential of the carbamate fungicide mancozeb. J Environ Pathol Toxicol Oncol.
2001;
20
:
127-131
.
-
N.
Titenko-Holland,
R.A.
Jacob,
N.
Shang,
A.
Balaraman,
M.T.
Smith.
Micronuclei in lymphocytes and exfoliated buccal cells of postmenopausal women with dietary changes in folate. Mutat Res.
1998;
417
:
101-114
.
-
P.E.
Tolbert,
C.M.
Shy,
J.W.
Allen.
Micronuclei and other nuclear anomalies in buccal smears: A field test in snuff users. Am J Epidemiol.
1991;
134
:
840-850
.
-
J.W.
Van Der Gulden,
P.F.
Vogelzang.
Farmers at risk for prostate cancer. Br J Urol.
1996;
77
:
6-14
.
-
World Health Organization.
Technical report series: Toxic hazards of pesticides to man. Geneva.
1962;
:
227
.
-
D.
Zeljezic,
V.
Garaj-Vrhovac.
Chromosomal aberration and single cell gel electrophoresis (Comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis.
2001;
16
:
359-363
.
-
D.
Zeljezic,
V.
Garaj-Vrhovac.
Sister chromatid exchange and proliferative rate index in the longitudinal risk assessment of occupational exposure to pesticides. Chemosphere.
2002;
46
:
295-303
.
-
T.
Zheng,
S.H.
Zahm,
K.P.
Cantor,
D.D.
Weisenburger,
Y.
Zhang,
A.
Blair.
Agricultural exposure to carbamate pesticides and risk of non-Hodgkin lymphoma. J Occup Environ Med.
2001;
43
:
641-649
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 03 (2014)
Page No.: 78-84
Published on: 2014-06-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4527 times
- Download PDF downloaded - 1006 times
- View Article downloaded - 6 times
 Biomedpress
Biomedpress