Calretinin negative Paratesticular mesothelioma: a rare case report
Abstract
Malignant mesothelioma of the tunica vaginalis is rare. The etiology is unclear. Exposure to asbestos is attributed as one of the risk factor. It is commonly seen in the elderly age group with hydrocele as the presenting complaint. Diagnosis is usually confirmed following histopathological examination, marker studies by immunohistochemistry and electron microscopy. We present a case in a 54 year old male who presented with left scrotal swelling. Immunohistochemically the tumor cells were positive for WT1, keratins, EMA, vimentin and negative for calretinin, desmin, CEA and actin. Ours is a rare case of paratesticular mesothelioma with calretinin negative.
Introduction
Paratesticular mesotheliomas are rare tumors and constitute 0.3 – 1.4% of all malignant mesotheliomas Bisceglia et al., 2010. The usual age at presentation is between sixth to eighth decade. The causative factors are asbestos exposure, long standing hydrocele and trauma Bisceglia et al., 2010Fonseca LG, 2014. It can involve the tunica vaginalis, spermatic cord or the epididymis with the former being most commonly affected Mrinakova et al., 2012. Diffuse malignant mesotheliomas are very aggressive tumors with poor prognosis because of a high rate of recurrence/ local and distant metastases and death Trpkov et al., 2011.
Case Report
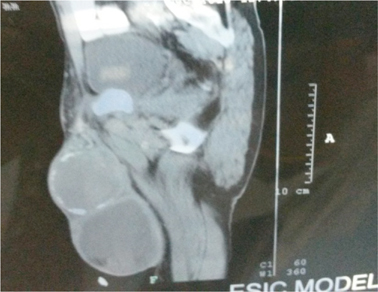
A 54 year old male presented with history of painless left scrotal swelling since 6 months. There was no past history of asbestos exposure, hydrocele or trauma. A clinical diagnosis of testicular tumor was made. Ultrasonography revealed a large collection in the left tunica and scrotal wall with inguinal lymphadenopathy suggesting the possibility of Koch’s, however malignancy cannot be ruled out. CT scan revealed grossly enlarged left testis with heterogeneous density with areas of necrosis in it and peripheral curvilinear calcification along the left testicular capsule suggestive of carcinoma testis ( Figure 1 ). Encysted hydrocele in the tunica vaginalis on the left side below the left testicular mass was noted. Moderately enhancing multiple enlarged left inguinal lymph nodes along with external iliac group of lymph nodes with a few mildly enlarged right inguinal group of lymph nodes was noted suggesting the possibility of metastasis. Serum alfa-feto protein and β-Human Chorionic Gonadotrophin were within normal limits.
Fine needle aspiration cytology (FNAC) of scrotal swelling showed moderate cell yield consisting of round to polygonal tumour cells having vesicular nucleus, coarse granular chromatin, some showed prominent nucleoli, moderate amount of eosinophilic cytoplasm and well defined cell border in a background of hemorrhage and necrosis. A diagnosis of malignant lesion was offered. FNAC of right and left inguinal lymph nodes revealed similar above described cells suggesting metastatic deposits. Left radical orchidectomy was performed.
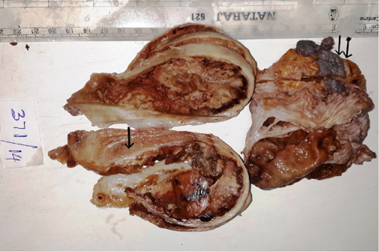
Grossly specimen consisted of a globular gray white to gray brown capsulated soft tissue mass, measured 12x7x6 cms with spermatic cord and thickened hydrocele sac. Cut section revealed a cystic lesion with gray white to gray brown friable tissue occupying the cystic space. The cyst wall thickness was variable from 0.5 to 2.5 cms with one area showed normal testicular tissue measuring 3×1.5 cms ( Figure 2 ). At focal areas the cyst wall showed yellowish speckled areas of calcification.
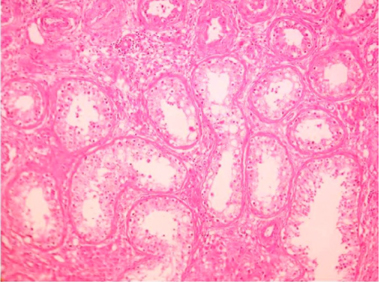
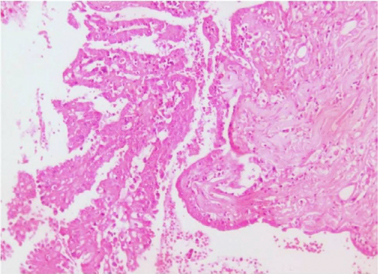
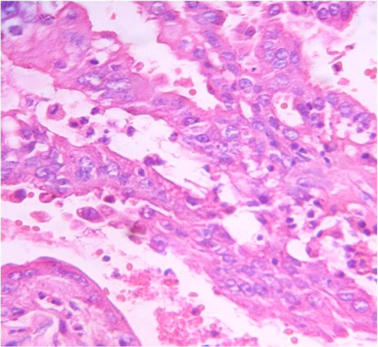
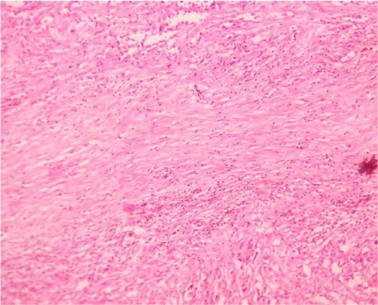
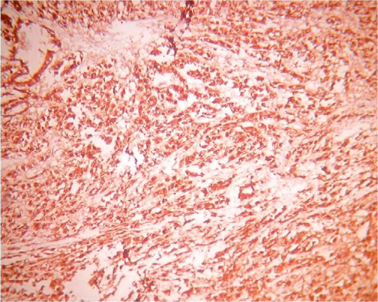
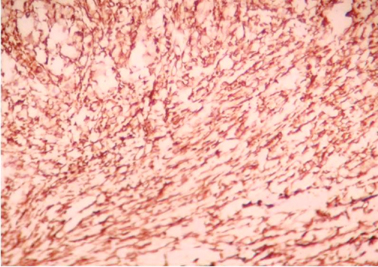
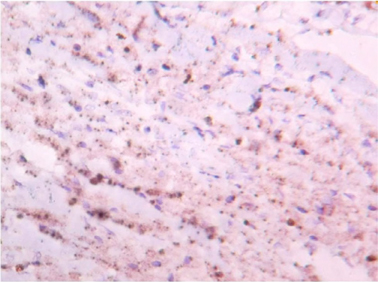
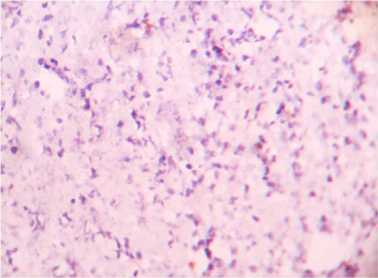
Microscopically sections from testis and epididymis were within normal limits ( Figure 3 ). tumor proper showed thick fibrocollagenous cyst wall lined by Single layer of cuboidal to columnar hyperplastic mesothelial cells and at places these cells were stratified forming papillary structures. The tumour cells were also seen as sheets and in alveolar pattern. The tumour cells were predominantly polyhedral having hyperchromatic bizarre vesicular nucleus, prominent nucleoli, scanty to moderate eosinophilic cytoplasm with ill-defined cytoplasmic margin ( Figure 4 & Figure 5 ) . Focal areas of hemorrhage and necrosis were seen. Focal areas showed spindle shaped tumor cells with pleomorphic nuclei arranged in interlacing fascicles ( Figure 6 ). The cyst wall showed areas of osseous metaplasia, hemorrhage, necrosis, calcification, macrophages and foreign body giant cell reaction. Sections studied from spermatic cord and scrotal skin was within normal limits. Immunohistochemistry (IHC) showed that the tumor cells were diffusely positive for EMA, vimentin, WT1 ( Figure 7 & Figure 8 ); focally positive for CK5/6 and negative for calretinin, desmin, CD20, CEA and actin ( Figure 9 & Figure 10 ). A final diagnosis of biphasic variant of paratesticular mesothe-lioma was offered. Patient was discharged against medical advice and lost for follow-up.
Discussion
Paratesticular mesothelioma is a rare tumour and was first described by Barbera and Rubino in 1957. It constitute less than 1% of all malignant mesotheliomas with less than 250 cases being reported tili date Bisceglia et al., 2010. The usual occurrence is between 6th to 8th decade of life and rarely occurs in patients under 20 years of age. The causative factor as for other mesotheliomas is said to be exposure to asbestos with 30-40% cases having positive past history. Other factors implicated are long Standing hydrocele and trauma Bisceglia et al., 2010Fonseca LG, 2014. It can involve tunica vaginalis, spermatic cord or epididymis with the former being most commonly affected. Both sides can be equally affected but bilateral involvement is rare Mrinakova et al., 2012Trpkov et al., 2011. In the present case the patient was 54 years presented with no significant.
The most common presenting Symptom is scrotal enlargement and less commonly testicular pain and mass Esen et al., 2012. Clinical diagnosis is usually made as hydrocele of unknown origin or testicular tumor. The clinical differential diagnosis include hydrocele, testicular tumors, cysts of testicular parenchyma, cysts of tunica albuginea, reactive mesothelial hyperplasia, adenomatoid tumor, adenocarcinoma of the epididymis, testicular metastatic carcinoma
Doppler ultrasonography helps at arriving at a near possible diagnosis. CT and MRI should be done to rule out lymph node involvement and distant metastasis. Technetium-99m scintigraphy also helps in identifying the primary tumor. Serum tumor markers are usually negative. Ultrasound guided FNAC may provide a clue and help at arriving at a diagnosis Bisceglia et al., 2010Mrinakova et al., 2012. In the present case the patient presented with painless scrotal swelling and the clinical diagnosis was testicular neoplasm. Ultrasound diagnosis was Koch’s. CT scan diagnosis was testicular Carcinoma with probably metastatic deposits in inguinal lymph nodes. Serum tumour markers were not contributory. FNAC of offered a diagnosis of malignant lesion of scrotal swelling with metastatic deposits in bilateral inguinal lymph nodes.
On gross examination small gray white exophytic nodules are usually seen on the visceral tunica vaginalis. Many times it presents as hematocele with raged and necrotic areas. Microscopically three variants are described; pure epithelial type, pure spindle cell type and mixed type (biphasic variant) with epithelial type being the most common. Three architectural patterns have been described in the literature; papillary, tubulopapillary and multicystic Mrinakova et al., 2012.
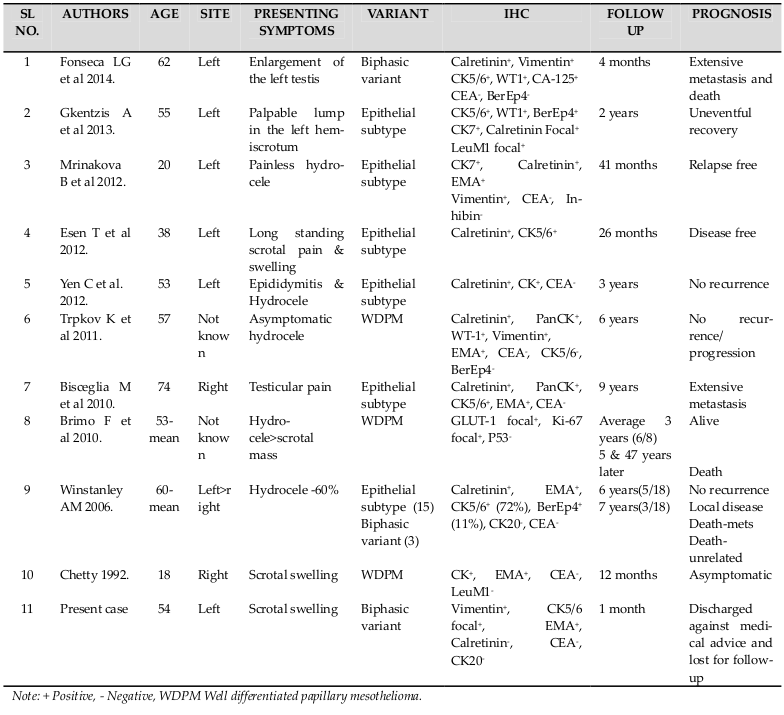
Features favoring malignancy include infiltration into deeper tissues, cytological atypia, prominent cell groupings and necrosis. The biphasic variant is described as having both interwoven bundles of spindle shaped tumor cells with foci of osseous and cartilaginous metaplasia and epithelial-like cells arranged in papillary or pseudoacinar pattern Trpkov et al., 2011. Electron microscopy is confirmative with presence of long slender microvilli on the apical surface of tumor cells Rosai, 2012Winstanley et al., 2006. Immunohisto-chemically, 100% of the epithelial, 70% of the biphasic variants and 60% of the pure spindle cell variants are found to be Calretinin positive. Cytokeratin 5/6 postivity is present in 100% of the epithelial variants and 10% of biphasic mesotheliomas. EMA, WT1 and vimentin have been found to be positive frequently. Tumor cells are negative for CEA, CK 20 and Leu Ml Hammar, 2006Winstanley et al.,2006. In the present case, grossly the lesion presented as cystic lesion with marked areas of hemorrhage and necrosis. Microscopically it was a mixed variant with papillary and acinar pattern. The IHC findings are shown in Table 1 .
The prognosis of this tumor is poor with median overall survival reported is 24 months. Local recurrence and death have been frequently reported. Extensive lymph node metastasis has been noted. The most common lymph nodes involved are retroperitoneal aorto-caval, inguinal and pelvic lymph nodes Fonseca LG, 2014Gkentzis et al., 2013. The clinico-pathological features with prognosis in various studies are shown in Table 1 . Radical orchidectomy is the surgery of choice; chemotherapy not being very effective. Aggressive surgical resection at the early stages has been found to have a good prognosis. Younger age, well differentiated tumor morphology, multicystic pattern are reported to have better prognosis. The aggressive behavior of the tumor calls for a regular and thorough follow up Brimo et al., 2010Chetty, 1992Fonseca LG, 2014Yen et al., 2012. In the present case the patient presented with bilateral inguinal lymph node deposits, underwent radical orchidectomy and was lost for follow-up.
Conclusion
To conclude, Paratesticular mesothelioma is rare malignant mesotheliomas and has a high rate of recurrence and metastasis. We present this case for its rarity.
Abbreviations
CA-125: Cancer antigen/ Carcinoma antigen/ carbohydrate antigen 125, CD: Cluster differentiation, CEA: Carcino-embroyonic antigen; CK: Cytokeratin; CT: Computerised tomography; EMA: Epithelial membrane antigen; FNAC: Fine needle aspiration cytology; GLUT 1: Glucose transporter 1; IHC: Immunohistochemistry; MRI: Magnetic resonance imaging; N:C: Nuclear:Cytoplasmic ratio; WT1: Wilm’s tumour 1.
Authors’ contributions
Ankitha Hebbar – Data collection, writing Script, photography. Kalyani Raju – concept, data collection, revision of manuscript, editing manuscript, selecting gross and microphotograph images. Srinivas Murthy V – editing the manuscript
References
-
M.
Bisceglia,
D.B.
Dor,
I.
Carosi,
M.
Vairo,
G.
Pasquinelli.
Paratesticular mesothelioma. Report of a case with comprehensive review of literature. Advances in anatomic pathology.
2010;
17
:
53-70
.
-
F.
Brimo,
P.B.
Illei,
J.I.
Epstein.
Mesothelioma of the tunica vaginalis: a series of eight cases with uncertain malignant potential. Modern pathology: an official Journal of the United States and Canadian Academy of.
2010;
Pathology
:
Inc 23, 1165-1172
.
-
R.
Chetty.
Well differentiated (benign) papillary mesothelioma of the tunica vaginalis. Journal of clinical pathology.
1992;
45
:
1029-1030
.
-
T.
Esen,
O.
Acar,
K.
Peker,
K.
Sarman,
A.
Musaoglu,
A.
Tefekli.
Malignant mesothelioma of the tunica vaginalis: presenting with intermittent scrotal pain and hydrocele. Case reports in medicine.
2012;
2012
:
189-170
.
-
M.D.
Fonseca LG,
Aguiar FN
Takahashi TK.
Malignant Paratesticular Mesothelioma. Autopsy Case Rep.
2014;
4
:
45-51
.
-
A.
Gkentzis,
K.
Sawalem,
J.
Husain.
An unusual case of paratesticular mesothelioma on the site of previously excised epididymal adenomatoid tumour. International journal of surgery case reports.
2013;
4
:
460-462
.
-
Hammar.
Immunohistochemistry of lung and pleural neoplasms. In Diagnostic Immunohistochemistry D. D., ed. (China: Elsevier).
2006;
:
329-403
.
-
B.
Mrinakova,
D.
Ondrus,
K.
Kajo,
M.
Kunderlik,
M.
Tkacova,
M.
Ondrusova.
Paratesticular mesothelioma in young age. Case report. Klinicka onkologie: casopis Ceske a Slovenske onkologicke spolecnosti.
2012;
25
:
290-293
.
-
Rosai.
Male reproductive system - Testiculae adnexa. In Rosai and Ackerman’s Surgical Pathology (Elsevier).
2012;
:
1375-1382
.
-
K.
Trpkov,
R.
Barr,
A.
Kulaga,
A.
Yilmaz.
Mesothelioma of tunica vaginalis of “uncertain malignant potential” - an evolving concept: case report and review of the literature. Diagnostic pathology.
2011;
6
:
78
.
-
A.M.
Winstanley,
G.
Landon,
D.
Berney,
S.
Minhas,
C.
Fisher,
M.C.
Parkinson.
The immunohistochemical profile of malignant mesotheliomas of the tunica vaginalis: a study of 20 cases. The American journal of surgical pathology.
2006;
30
:
16
.
-
C.H.
Yen,
C.T.
Lee,
C.J.
Su,
H.C.
Lo.
Malignant mesothelioma of the tunica vaginalis testis: a malignancy associated with recurrent epididymitis?. World journal of surgical oncology.
2012;
10
:
238
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 04 (2014)
Page No.: 106-111
Published on: 2014-09-07
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4159 times
- Download PDF downloaded - 1069 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress