Abstract
Introduction: Guttate is a type of psoriasis in which patients are sensitive to Streptococcus pneumoniae throughout innate immune responses. During the inflammation, tumour necrosis factor alpha (TNF-α), a well-known pro-inflammatory cytokine, is expressed; meanwhile interleukin 10 (IL-10) and heparin-binding EGF-like growth factor (HB-EGF), which are capable of inhibiting transcription of the TNF-α gene, are also prominent. Furthermore, HB-EGF only impacts fibroblasts and keratinocytes which promote psoriatic lesions. In this study, we looked for differences of TNF-α, IL-10 and HB-EGF expression between a psoriatic patient and a non-psoriatic relative.
Methods: To achieve our target, peripheral blood mononuclear cells (PBMCs) expressing LPS receptors or CD14 (CD14+ cells) derived from a guttate patient, and the donor’s father (without psoriatic symptoms), were activated for 7 days by a lysate of Streptococcus pneumoniae for 24 hours before being harvested.
Results: Results showed detectable mRNAs of TNF-α, IL-10 and HB-EGF from isolated CD14+ cells of guttate patient were more intensive expression than the non-psoriatic one at 24 hours after engaging the bacterial components. In addition, transcription of HB-EGF gene from the guttate patient was maintained over 168 hours, while its mRNA level from the non-psoriatic volunteer was only expressed within 24 hours.
Conclusion: Finally, in initial results of inflammatory effects between strains, the Streptococcal lysate was seen to have stronger immune responses than the Staphylococcal lysate on the immune cells of the guttate psoriasis.
Introduction
Psoriasis is a chronic cutaneous inflammation in dermatology. To date, there have been a possibility of 40 susceptibility loci identified from various forms of psoriasis Baurecht et al., 2015Tsoi et al., 2012. The vast majority of these loci encode major histocompatibility complex (MHC), pro-inflammatory cytokines and nuclear transcriptional factors in both innate and adaptive immune responses Jordan et al., 2012Nair et al., 2006Sato et al., 2015. Those elements are abnormally increased in gene expression in active peripheral blood mononuclear cells. Gervin et al. (2012) illustrated that there was a correlation between DNA methylation and expressions of IL13 and TNFSF11 genes in CD4 lymphocytes of monozygotic twins deferring psoriatic phenotype Gervin et al., 2012. Interestingly, a streptococcal infection in the throat could lead to an exacerbation of almost all lesions on psoriasis patients during inflammation Gudjonsson et al., 2003. According to these results, forming a psoriatic lesion might require environmental stimulators to activate cytokines involved in immune responses. Therefore, psoriasis might be a consequence of imbalance of inflammatory homeostasis.
Tumour necrosis factor alpha (TNF-α) is one of the first pro-inflammatory cytokines produced in innate immune response. Typical immune cells such as T helper 17 lymphocytes (Th17), dendritic cells (DCs) and macrophages are stimulated throughout trauma by pathogens, especially lipopolysaccharide (LPS) of bacteria Evans et al., 2009Haider et al., 2008. When LPS receptors or CD14 on these cells are activated, inflammation is triggered by the NF-κB pathway. As results of LPS induction, the activated immune cells immediately secrete TNF-α as well as other pro-inflammatory cytokine which probably lead to IL17, IL24, and IL33 production from keratinocytes Balato et al., 2012Chiricozzi et al., 2011Kumari et al., 2013Volpe et al., 2014. Furthermore, mutations in loci coding the transcriptional factors may initiate lesions. For example, the A20 mutant in plaque psoriasis could enhance TNF-α expression through the NF-κB pathway Evans et al., 2004Shembade et al., 2010. Characteristically, when a large number of psoriatic lesions derive from skin injuries, it is known as the Koebner phenomenon Arias-Santiago et al., 2013Thorarensen et al., 2015.
Moreover, the epidermal growth factors (EGFs) may play a key role in psoriasis. In fact, there are three predominant EGFs, including heparin-binding EGF-like growth factor (HB-EGF), transforming growth factors (TGFs) and amphiregulin (AREG), which are involved in psoriasis. In post-inflammation, the presence of TGF-β and AREG prevent the transcription of inflammatory cytokine genes in immune cells at damaged skin. In addition, these two factors have similar anti-inflammatory function as IL-10, activating M2 macrophages and inflammatory suppression Gratchev et al., 2008Inoue et al., 2014Maheshwari et al., 2011. Similarly, HB-EGF plays a potential key role for common psoriatic types via epidermal growth factor receptors (EGFRs). It induces EGFRs in response to the hyperproliferation, hyperdifferentiation, or hyperplasia of keratinocytes Johnson et al., 1993. Moreover, expression of HB-EGF in human gastric carcinoma cells is also influenced by activation of the NF-κB pathway Baek et al., 2008. Some mutations in the HB-EGF gene in animal models can lead to psoriasis-like phenotype Poumay and De Rouvroit, 2012.
This study will observe the expression of TNF-α, IL-10 and HB-EGF in peripheral blood mononuclear cells expressing CD14+ (CD14+ cells) from a patient with guttate psoriasis, and his father without psoriasis, in a 7-day period. Additionally, the inflammatory effects of lysates of Streptococcus pneumoniae and Coagulase-negative Staphylococci on the CD14+ cells of guttate psoriasis were compared.
Materials-Methods
Study design
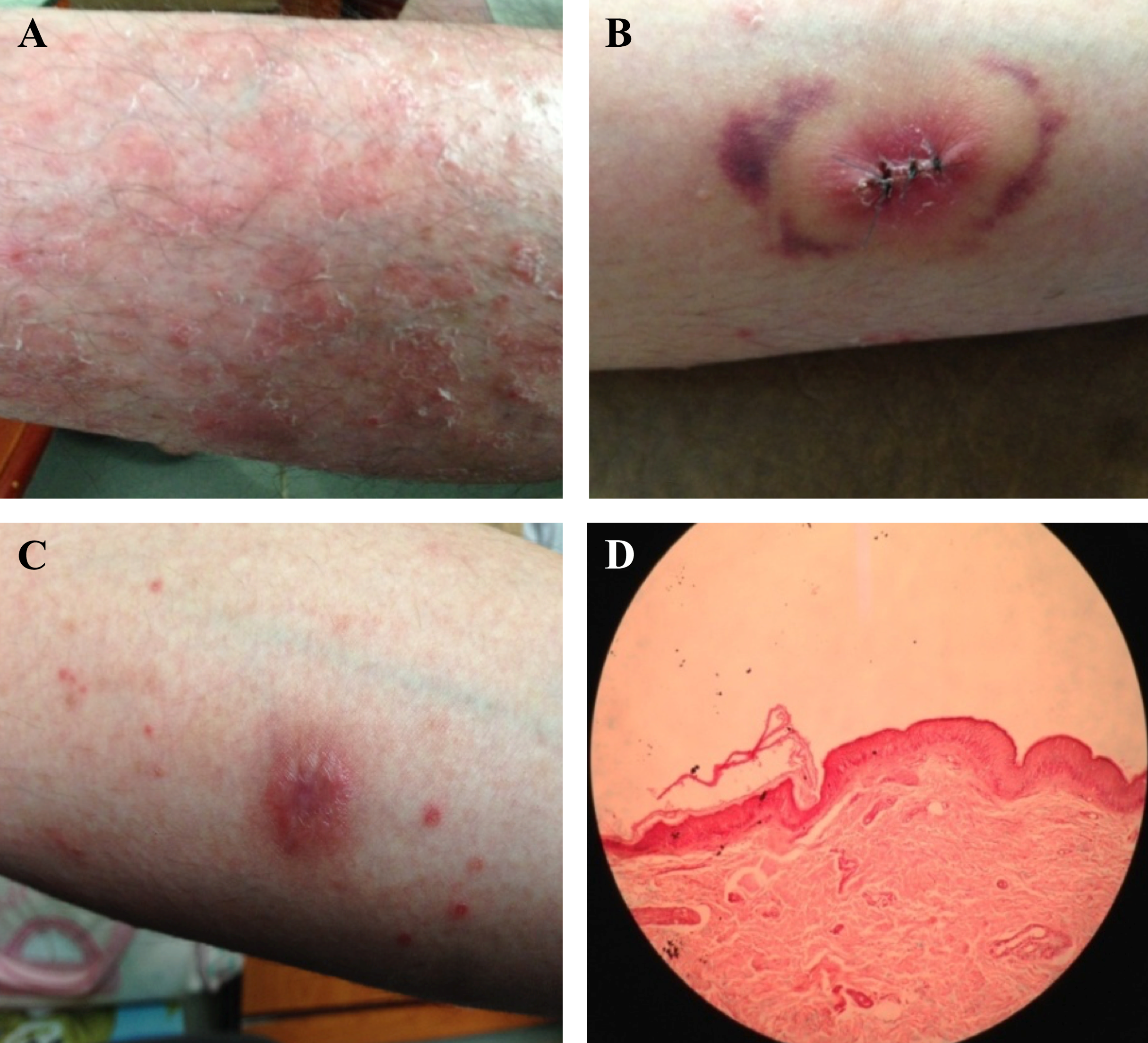
Two volunteers (a 34-year-old patient with guttate psoriasis, and his father with non-psoriasis as a control) agreed and signed the consent form to enroll the study, which was approved by the Ethic Medical Research Committee, University of Medicine Pham Ngoc Thach, Vietnam, according to the Declaration of Helsinki. Major criteria were: the psoriatic patient was diagnosed at the Ho Chi Minh City Hospital of Dermato–Venereology, Vietnam; PASI >10%; psoriatic symptoms for over 10 years; neither systemic therapy nor antiviral drugs were applied; no acute bacterial infections nor immune suppression, and no severe renal or deficient immune diseases or cancers ( Figure 1 ).
Peripheral blood isolation
Each volunteer had 6mL of peripheral venous blood taken once per two-weeks and the process was repeated in three times. All peripheral blood samples were collected into sterile lithium heparin tubes (Hong Thien My, Ho Chi Minh City, Vietnam), which were stored at approximately 4ºC and transported to the laboratory within 2 hours.
Bacterial lysis
Streptococcus pneumoniae strain (ID: ATCC®49619™) and Coagulase-negative Staphylococci strain which were kindly provided by the Department of Microbiology, University of Medicine Pham Ngoc Thach. Generally, those strains were re-confirmed as Streptococcus pneumoniae and Coagulase-negative Staphylococci strains at the Department of Microbiology. To preserve functional proteins, the bacteria were lysated at extremely low temperature. In general, an individual colony of each strain was inoculated and dipped into the brain-heart infusion (BHI) medium (Merck, Darmstadt, Germany) at 37ºC overnight. Subsequently, this overnight medium was centrifuged to obtain pellet (around 23 mg). The pellet was thawed by liquid nitrogen and rethawed at 37ºC five times before being mixed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH of 7.2.) supplemented with 1% of penicillin/ streptomycin (Sigma-Aldrich, St. Louis, MO, USA), made up to a final volume of 500 mL and were stored at -20°C for further steps.
CD14+ cell preparation and inflammatory induction
Human peripheral blood mononuclear cells (PBMCs) from the heparinised venous blood of the participants were divided into two groups: guttate and control, respectively. In each group, PBMCs were sorted by Ficoll-PaqueTM PLUS (GE Healthcare, Uppsala, Sweden). Then, they were washed and resuspended three times in cold RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 1% of penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Next, the 107 mononuclear cells in each group were incubated with 20 μL of CD14 MicroBeads, before the CD14 MicroBead-bound mononuclear cells (CD14+ cells) in the cell suspension were positively selected by magnetic activated cell sorting technology (Miltenyi Biotech, Bergisch Gladbach, Germany) in accordance with the manufacturer’s instructions. Finally, 104 cells of isolated CD14+ cells were aliquoted into seven samples for each group and were cultured with RPMI 1640 medium at 37°C, 5% CO2 overnight. For stimulation of inflammation, 10μL of the thawed Streptococcal or Staphylococcal lysate was added into six samples which were harvested at hours 1, 24, 48, 72, 120, 168, while the remaining sample was not. The RPMI 1640 medium was replaced every 24 hours.
RNA extraction and complementary DNA synthesis
Total RNA was extracted from activated CD14+ cells by TriPure (Roche, Mannheim, Germany) following the manufacturer’s instructions. First strand complementary DNA (cDNA) was synthesised from total RNA with Random Primer Mix (New England BioLabs, MA) by reverse transcription-polymerase chain reaction (RT-PCR), according to a standard cDNA synthesis protocol of the manufacturer. The procedure of RT-PCR was conducted at 42°C for 60 min prior to inactivating at 80°C for 5 min. The cDNA products were stored at -20°C.
Analysis of mRNA gene expression
To observe mRNA level of TNF-α, IL-10, HB-EGF, and GAPDH, their primers were created using the Primer3 (version 0.4.0) and the published genetic sequences from the National Center for Biotechnology Information (NCBI). Reactions in PCR were performed with the One Taq® 2X Master mixed and Standard Buffer kit (New England BioLabs, MA, USA) in accordance with the manufacturer’s directions. Each reaction was optimized with 950 ng of cDNA template using the following primers: TNF-α forward: 5’-ACAAGCCTGTAGCC-CATGTT-3’, TNF-α reverse: 5’-AAAGTAGACCTGCCCAGACT-3’ (GenBank: NM_000594.3); IL-10 forward: 5’-TGCCTTCAGCAGAGTGAAGA-3’, IL-10 reverse: 5’-GGTCTTGGTTCTCAGCTTGG-3’ (GenBank: NM_000572.2); HB-EGF forward: 5’-GGTGGTGCTGAAGCTCTTTC-3’, HB-EGF reverse: 5’-GCTTGTGGCTTGGAGGATAA-3’ (GenBank: NM_001945.2); GAPDH forward: 5’-GAGTCAACGGATTTGGTCGT-3’, GAPDH reverse: 5’ - TTGATT-TTGGAGGGATCTCG-3’ (GenBank: NM_001289746.1). All primers were purchased from the Integrated DNA Technologies Pte Ltd (Singapore). Reactions were run on the Eppendorf® Mastercycler for 1 min at 95°C; and 30 cycles of 15 sec at 95°C followed by 60 sec at 50°C (TNF-α, IL-10 and HB-EGF) or 60°C (GAPDH). All PCR products were run on electrophoresis in 1.5% TBE agarose gel, and the results were analyzed with the UVP-GelDoc-It Imaging System to determine a relative amount of cDNA bands. Generally, this software compares the brightness of bands of interest with the 500 bp band of the Quick-Load® Purple 2-Log DNA Ladder (0.1 - 10.0 kb) (New England BioLabs, MA, USA), and then 12.4 ng/μL of DNA was multiplied with the ratios of brightness between each segment of interest and 500bp band. A 623bp band from IL-10 PCR product was sequenced by a commercial DNA sequencing service (First BASE Laboratories Sdn Bhd, Malaysia). Finally, the Unipro UGENE software (Version 1.26.0) was used for cDNA sequence analysis.
Date analysis
Statistical calculation for intensity of electrophoretic bands, which were compared different gene expression between guttate and control group in RT-PCR analysis, was conducted by two-way ANOVA with replication using Microsoft Excel software (Microsoft Inc, WA, USA). Standard error of the mean was utilized for error bars on three graphs.
Results
Streptococcal lysate and inflammation from activated CD14+ cells of guttate psoriasis
To monitor innate immune response of CD14+ cells from a patient with guttate psoriasis in vitro, the streptococcal lysate was used for this purpose. Further, the non-stimulated cell sample (0h) was used to verify all CD14+ cells which were not activated from previous steps. This ensures that those cells will naturally respond to the pathogen as well as exposing abnormal transcriptions between guttate and control groups. Besides, FBS was not supplemented into the RPMI 1640 medium because it contains several elements which could overlap with the transcriptional effects of HB-EGF.
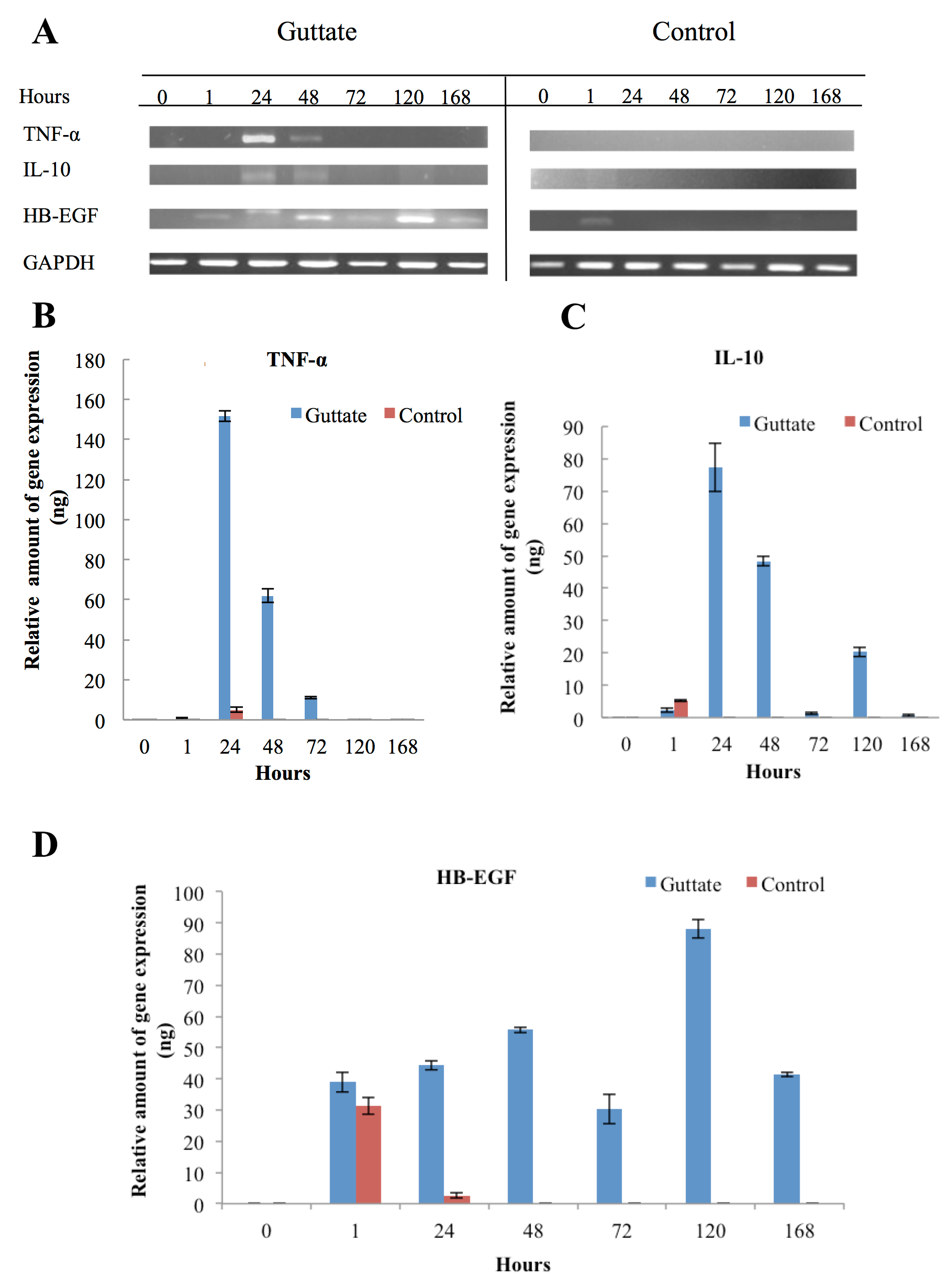
There were no cDNA signals of TNF-α, IL-10 and HB-EGF from the CD14+ cells in the non-stimulated samples (0) from both groups (psoriasis and control). After samples were stimulated by the lysate of Streptococcus pneumoniae, TNF-α, IL-10 and HB-EGF gene transcription occurred in the first few hours until hours 168. Regarding the TNF-α signal, it occurred around 24 hours. However, the level of TNF-α mRNA in CD14+ cells of guttate was over 50-folds stronger than that of control approximately 153 ng and 3 ng of cDNA, respectively. In addition to the inflammatory response from the CD14+ cells, the signal of TNF-α from guttate continued to persist for the next 24 hours (48h) and there was no band at hour 72 (72h). On the other hand, a weak signal was detected from the 24-hour sample of control and no signal was seen in the remaining five samples ( Figure 2A,B ).
The IL-10 and HB-EGF gene expressions of more prolonged to 120 and 168 hours, respectively. Although IL-10 is a well-known anti-inflammatory cytokine, our results revealed that the mRNA of IL-10 and TNF-α from guttate and control groups were simultaneously expressed around the first 24 hours (24h). Nevertheless, the CD14+ cells of the control group expressed the IL-10 gene in the first hour (1h), while expression of this anti-inflammatory cytokine from the guttate group was established around 24 hours to 120 hours (120h).
Subsequently, downregulation of this cytokine proceeded to occur over the next 48 hours ( Figure 2A,C ).
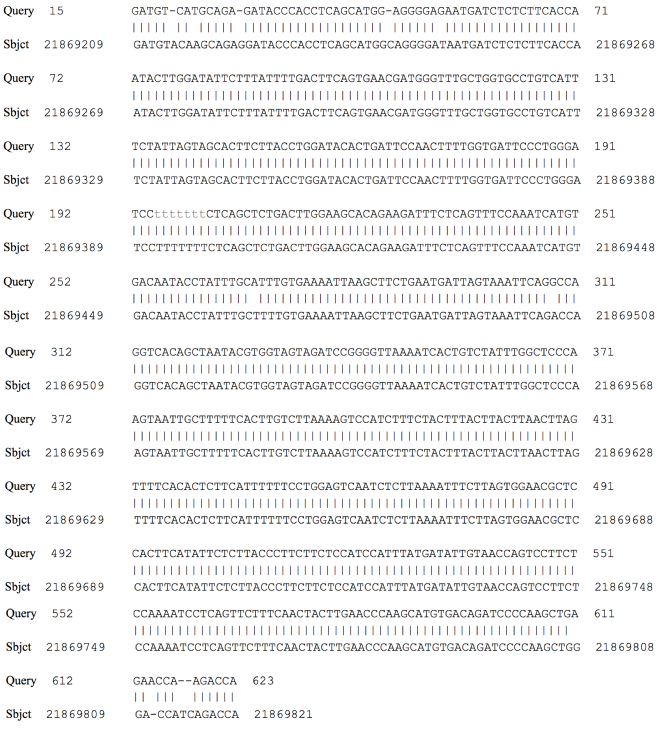
Similarly, transcription of the HB-EGF gene also progressed in the first hour for the two groups. The CD14+ cells of the control group showed weak expression at nearly 24h. Nonetheless, for the guttate group, this growth factor showed a gradual increasing expression before reaching a peak of gene expression at about 120 hours and persisting to over 168 hours ( Figure 2A,D ). Interestingly, regarding the IL-10 PCR product, there was an unknown band of approximately 623bp. The date of cDNA sequencing were compared with the database from NCBI throughout the BLAST programme. It showed that the sequences were homologous to chromosome 2 and there are two open reading frames (ORFs) in the sequences ( Figure 3 ).
The inflammatory stimuli of Streptococcus pneumoniae and Coagulase-negative Staphylococci on CD14+ cells of guttate
To understand the transcriptional effects of topical bacteria on the innate immune response, Coagulase-negative Staphylococci (Stap) was chosen because it pertains to human microbiota on skin. Our results demonstrated that this strain partly affects the CD14+ cells of the guttate group. The expressions of TNF- α, IL-10 and HB-EGF only occurred in 24 hours, and the mRNA level was probably lower than the yields obtained from Streptococcus pneumoniae (Strep)-stimulated CD14+ cells ( Figure 4 ).
Regarding the induction of inflammation on CD14+ cells by Coagulase-negative Staphylococci, the staphylococcal lysate was insufficiently strong to cause strong gene expressions of TNF-α, IL-10, and HB-EGF, in comparison with the streptococcal lysate. Additionally, mRNA expression of IL-10 and HB-EGF in the guttate group did not persist as with strep stimulation, and they were similar to the control group stimulated by streptococcal lysate. Therefore, CD14+ cells in the guttate psoriatic patient are less sensitive to the staphylococcal components in react with a strong immune response. In fact, streptococcal infection leads to aggravation of the vast majority of lesions of patients with psoriasis, whilst skin damage only causes a topical inflammatory response.
Discussion
The guttate psoriasis patient was sensitive to the sore throat, especially Streptococcus pneumoniae. This strain is one of the local bacteria of the upper respiratory tract which plays an important role in the lesional exacerbation. Previous studies have shown that psoriatic lesions can trigger inflammation via LPS, and that Strep possibly enhance the release of pro-inflammatory cytokines from CD4+ T cells and macrophages through the Streptococcal M6 protein Johnston et al., 2004Wang et al., 2006. However, in psoriasis, over-expression of mononuclear cells causes secretion of TNF-α, which significantly influences the large number of lesions. In this study, the signals of TNF-α, from the guttate psoriatic patient were more strongly expressed than the control (with non-psoriasis), although both participants were homologous in up to 50% of their genetic material. This means that suppressing gene expression of TNF-α in psoriatic patients less effective than normal people. Furthermore, approximately 160 genes involve inflammation such as S100A7, SerpinB4, and DEFB4 could be up-regulated by the TNF /IL-17 induction Chiricozzi et al., 2011. This might impact on immune cells which regulate inflammation through TNF-α expression. Additionally, the role of IL-10 is to suppress transcription of the TNF-α gene, but our results of these cytokines showed concomitant expression in the early 24 hours. In fact, the majority of CD14+ cells as macrophages will release IL-10 to suppress transcript level of TNF-α through the LPS receptor induction Wang et al., 2014. Saraiva et al. (2005) showed that there is a tight correlation between LPS-activated NF-κB complex and signal transduction of IL-10 through its promoter Saraiva et al., 2005. Moreover, several studies have demonstrated that IL-10 production can be possibly up-regulated from 2 to 8 hours by apoptotic neutrophils, proopiomelanocortin peptides, or CD23 activation Bhardwaj et al., 1996Byrne and Reen, 2002Dugas et al., 1996. Therefore, IL-10 production in CD14+ cells of guttate psoriasis corresponds to the signal transduction of TNF-α.
In this study, our hypothesis was that HB-EGF might play an important role in psoriatic pathway. This growth factor not only suppresses transcription of pro-inflammatory elements such as TNF-α gene Rocourt et al., 2007. Moreover, HB-EGF also induces hyperdifferentiation or hyperproliferation of keratinocytes the during the recovery of cutaneous injuries in psoriasis Shirakata et al., 2005. In our results of guttate, HB-EGF was produced in the first hour. In fact, we did not utilize FBS for cell culture so that other growth factors in FBS would not affect the LPS-induction of CD14+ cells. Despite differing signal transductions, it is probable that the streptococcal stimulus on the immune cells of the guttate group might activate a potential mediator which regulates the transcriptional systems of TNF-α as well as HB-EGF. Furthermore, the HB-EGF expression from guttate persisted to over 7 days (168 hours), while expression of the growth factor from the control group was just over 1 h after the inflammatory stimulation. The results of the control in this study correlates with research by Yoshizumi et al. (1992), which showed that TNF expression enhanced HB-EGF expression in 24 hours Yoshizumi et al., 1992. Conversely, the HB-EGF mRNA level in guttate continues to persist to over 168 hours. It is possible to systematically activate the NF-κB and PKB pathways causing the over-expression of HB-EGF Yotsumoto et al., 2010. In another clinical research of psoriasis, Anderson et al. (2010) demonstrated that serum HB-EGF and TNF-α concentration sustainably rise in patients with psoriatic plaques Anderson et al., 2010. This is substantial evidence that there is a correlation between induction of the NF- κB pathway and HB-EGF gene expression in psoriasis.
There are several limitations in this study. It lacks date of TNF-α, IL-10 and HB-EGF concentrations in cell culture and lacks identification of some transcriptional factors which may respond to expression of these elements. Subsequently, the sample size is too small and the pattern is only guttate without plaque psoriasis (the most common type of psoriasis). However, our data in this study will become a preliminary database for next studies. Regarding HB-EGF expression and the NF-κB pathway, the identification of transcriptional mediators in activated macrophages (CD14+, CD163+) of guttate and plaque psoriasis is ongoing.
Conclusion
In initial conclusion, the concomitant expression of TNF-α, IL-10 and HB-EGF, as well as abnormal transcription of the HB-EGF gene in a 168-hour period, may be indicators that CD14+ cells of guttate psoriasis are activated by LPS. In addition, streptococcal components are more pathogenic than staphylococcal ones. Control of HB-EGF gene transcription in immune cells is not only one of our targets improving the quality of life for psoriatic patients, but it is also probably applicable to immunotherapy and gene therapy for other autoimmune diseases and solid tumors in the future.
Abbreviations
AREG: amphiregulin
CD14: Cluster of differentiation 14
cDNA: complementary DNA
EGFR: Epidermal growth factor receptor
GADPH: Glyceraldehyde 3-phosphate dehydrogenase
HBEGF: Heparin-binding EGF-like growth factor
HE stain: Hematoxylin and eosin stain
IL: Interleukin
LPS: Lipopolysaccharide
MHC: Major histocompatibility complex
NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells
PASI: Psoriasis area severity index
PCR: Polymerase chain reaction
PKB: Protein kinase B
Th17: T helper 17 lymphocytes
TNF-α: Tumour necrosis factor alpha
TGFs: Transforming growth factors
Funding
This study was supported by the research fund of the University of Medicine Pham Ngoc Thach, Vietnam. The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
Author Contribution
All authors equally contributed on all experiments: designed the study; wrote the manuscript.
References
-
K.
Anderson,
S.
Petersson,
J.
Wong,
E.
Shubbar,
N.
Lokko,
M.
Carlström,
C.
Enerbäck.
Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. British Journal of Dermatology.
2010;
163(5)
:
1085-1089
.
View Article Google Scholar -
S.
Arias-Santiago,
M. J.
Espiñeira-Carmona,
J.
& Aneiros-Fernández.
The Koebner phenomenon: Psoriasis in tattoos. Canadian Medical Association Journal.
2013;
185(7)
:
585-585
.
View Article Google Scholar -
M. K.
Baek,
M. H.
Kim,
H. J.
Jang,
J. S.
Park,
I. J.
Chung,
B. A.
Shin,
Y. D.
Jung.
EGF stimulates uPAR expression and cell invasiveness through ERK, AP-1, and NF-κB signaling in human gastric carcinoma cells. Oncology Reports.
2008;
20
:
1569-1575
.
-
A.
Balato,
S.
Lembo,
M.
Mattii,
M.
Schiattarella,
R.
Marino,
A.
Paulis,
F.
Ayala.
IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Experimental Dermatology.
2012;
21(11)
:
892-894
.
View Article Google Scholar -
H.
Baurecht,
M.
Hotze,
S.
Brand,
C.
Büning,
P.
Cormican,
A.
Corvin,
R.
Fölster-Holst.
Genome-wide Comparative Analysis of Atopic Dermatitis and Psoriasis Gives Insight into Opposing Genetic Mechanisms. American Journal of Human Genetics.
2015;
96(1)
:
104-120
.
View Article Google Scholar -
R. S.
Bhardwaj,
A.
Schwarz,
E.
Becher,
K.
Mahnke,
Y.
Aragane,
T.
Schwarz,
T. A.
Luger.
Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. Journal of Immunology (Baltimore, Md.: 1950).
1996;
156
:
2517-2521
.
-
A.
Byrne,
D. J.
Reen.
Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. Journal of Immunology (Baltimore, Md.: 1950).
2002;
168(4)
:
1968-1977
.
-
A.
Chiricozzi,
E.
Guttman-Yassky,
M.
Suárez-Farinas,
K. E.
Nograles,
S.
Tian,
I.
Cardinale,
J. G.
Krueger.
Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. The Journal of Investigative Dermatology.
2011;
131(3)
:
677-687
.
View Article Google Scholar -
N.
Dugas,
I.
Vouldoukis,
P.
Bécherel,
M.
Arock,
P.
Debré,
M.
Tardieu,
B.
Dugas.
Triggering of CD23b antigen by anti-CD23 monoclonal antibodies induces interleukin-10 production by human macrophages. European Journal of Immunology.
1996;
26(6)
:
1394-1398
.
View Article Google Scholar -
H. G.
Evans,
N. J.
Gullick,
S.
Kelly,
C.
Pitzalis,
G. M.
Lord,
B. W.
Kirkham,
L. S.
Taams.
In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proceedings of the National Academy of Sciences of the United States of America.
2009;
106(15)
:
6232-6237
.
View Article Google Scholar -
P.
Evans,
H.
Ovaa,
M.
Hamon,
P.
Kilshaw,
S.
Hamm,
S.
Bauer,
T.
Smith.
Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. The Biochemical Journal.
2004;
378(3)
:
727-734
.
View Article Google Scholar -
K.
Gervin,
M. D.
Vigeland,
M.
Mattingsdal,
M.
Hammero,
H.
Nygard,
A. O.
Olsen,
R.
Lyle.
DNA methylation and gene expression changes in monozygotic twins discordant for psoriasis: Identification of epigenetically dysregulated genes. PLOS Genetics.
2012;
8(1)
:
e1002454
.
View Article Google Scholar -
A.
Gratchev,
J.
Kzhyshkowska,
S.
Kannookadan,
M.
Ochsenreiter,
A.
Popova,
X.
Yu,
L.
Gooi.
Activation of a TGF-β-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-β receptor II. Journal of Immunology (Baltimore, Md.: 1950).
2008;
180(10)
:
6553-6565
.
-
J.
Gudjonsson,
A.
Thorarinsson,
B.
Sigurgeirsson,
K.
Kristinsson,
H.
Valdimarsson.
Streptococcal throat infections and exacerbation of chronic plaque psoriasis: A prospective study. British Journal of Dermatology.
2003;
149(3)
:
530-534
.
View Article Google Scholar -
A. S.
Haider,
M. A.
Lowes,
M.
Suárez-Fariñas,
L. C.
Zaba,
I.
Cardinale,
A.
Khatcherian,
J. G.
Krueger.
Identification of cellular pathways of “type 1,” Th17 T cells, and TNF-and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. Journal of Immunology (Baltimore, Md.: 1950).
2008;
180(3)
:
1913-1920
.
-
M.
Inoue,
T.
Arikawa,
Y.-H.
Chen,
Y.
Moriwaki,
M.
Price,
M.
Brown,
M. L.
Shinohara.
T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. Proceedings of the National Academy of Sciences of the United States of America.
2014;
111(14)
:
5295-5300
.
View Article Google Scholar -
G. R.
Johnson,
B.
Kannan,
M.
Shoyab,
K.
Stromberg.
Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. The Journal of Biological Chemistry.
1993;
268
:
2924-2931
.
-
A.
Johnston,
J.
Gudjonsson,
H.
Sigmundsdottir,
T.
Love,
H.
Valdimarsson.
Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8+ T cells. Clinical and Experimental Immunology.
2004;
138(1)
:
83-93
.
View Article Google Scholar -
C. T.
Jordan,
L.
Cao,
E. D.
Roberson,
S.
Duan,
C. A.
Helms,
R. P.
Nair,
G.
Hayashi.
Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. American Journal of Human Genetics.
2012;
90(5)
:
796-808
.
View Article Google Scholar -
S.
Kumari,
M. C.
Bonnet,
M. H.
Ulvmar,
K.
Wolk,
N.
Karagianni,
E.
Witte,
R.
Toftgard.
Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity.
2013;
39(5)
:
899-911
.
View Article Google Scholar -
A.
Maheshwari,
D. R.
Kelly,
T.
Nicola,
N.
Ambalavanan,
S. K.
Jain,
J.
Murphy-Ullrich,
C.
Aprahamian.
TGF-β 2 Suppresses Macrophage Cytokine Production and Mucosal Inflammatory Responses in the Developing Intestine. Gastroenterology.
2011;
140(1)
:
242-253
.
View Article Google Scholar -
R. P.
Nair,
P. E.
Stuart,
I.
Nistor,
R.
Hiremagalore,
N. V.
Chia,
S.
Jenisch,
E.
. . . Christophers.
Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. American Journal of Human Genetics.
2006;
78(5)
:
827-851
.
View Article Google Scholar -
Y.
Poumay,
C. L.
De Rouvroit.
HB-EGF, the growth factor that accelerates keratinocyte migration, but slows proliferation. The Journal of Investigative Dermatology.
2012;
132(9)
:
2129-2130
.
View Article Google Scholar -
D. V.
Rocourt,
V. B.
Mehta,
G. E.
Besner.
Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. The Journal of Surgical Research.
2007;
139(2)
:
269-273
.
View Article Google Scholar -
M.
Saraiva,
J. R.
Christensen,
A. V.
Tsytsykova,
A. E.
Goldfeld,
S. C.
Ley,
D.
Kioussis,
O.
Anne.
Identification of a macrophage-specific chromatin signature in the IL-10 locus. Journal of Immunology (Baltimore, Md.: 1950).
2005;
175(2)
:
1041-1046
.
-
K.
Sato,
M.
Takaishi,
S.
Tokuoka,
S.
Sano.
Correction: Involvement of TNF-α Converting Enzyme in the Development of Psoriasis-Like Lesions in a Mouse Model. PLoS One.
2015;
10(4)
:
e0124989
.
View Article Google Scholar -
N.
Shembade,
A.
Ma,
E. W.
Harhaj.
Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science.
2010;
327(5969)
:
1135-1139
.
View Article Google Scholar -
Y.
Shirakata,
R.
Kimura,
D.
Nanba,
R.
Iwamoto,
S.
Tokumaru,
C.
Morimoto,
E.
Mekada.
Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. Journal of Cell Science.
2005;
118(11)
:
2363-2370
.
View Article Google Scholar -
S.
Thorarensen,
N.
Lu,
A.
Ogdie,
J.
Gelfand,
H.
Choi,
T.
Love.
OP0311 Physical Trauma is Associated with the Onset of Psoriatic Arthritis Among Psoriasis Patients. Annals of the Rheumatic Diseases.
2015;
74(Suppl 2)
:
190-191
.
View Article Google Scholar -
L. C.
Tsoi,
S. L.
Spain,
J.
Knight,
E.
Ellinghaus,
P. E.
Stuart,
F.
Capon,
J. E.
Gudjonsson.
Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nature Genetics.
2012;
44(12)
:
1341-1348
.
View Article Google Scholar -
E.
Volpe,
L.
Pattarini,
C.
Martinez-Cingolani,
S.
Meller,
M.-H.
Donnadieu,
S.I.
Bogiatzi,
M.I.
Fernandez,
M.
Touzot,
J.-C.
Bichet,
F.
Reyal.
Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. Journal of Allergy and Clinical Immunology.
2014;
134
:
373-381
.
-
B.
Wang,
Y.-H.
Rao,
M.
Inoue,
R.
Hao,
C.-H.
Lai,
D.
Chen,
M. L.
Shinohara.
Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nature Communications.
2014;
5
:
3479-3479
.
-
H.
Wang,
T.
Peters,
D.
Kess,
A.
Sindrilaru,
T.
Oreshkova,
N.
Van Rooijen,
M.
Wlaschek.
Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. The Journal of Clinical Investigation.
2006;
116(8)
:
2094-2105
.
View Article Google Scholar -
M.
Yoshizumi,
S.
Kourembanas,
D.
Temizer,
R.
Cambria,
T.
Quertermous,
M.-E.
Lee.
Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. The Journal of Biological Chemistry.
1992;
267
:
9467-9469
.
-
F.
Yotsumoto,
E.
Oki,
E.
Tokunaga,
Y.
Maehara,
M.
Kuroki,
S.
Miyamoto.
HB-EGF orchestrates the complex signals involved in triple-negative and trastuzumab-resistant breast cancer. International Journal of Cancer.
2010;
127(11)
:
2707-2717
.
View Article Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 4 No 11 (2017)
Page No.: 1733-1748
Published on: 2017-11-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 7130 times
- Download PDF downloaded - 1859 times
- View Article downloaded - 34 times
 Biomedpress
Biomedpress