Abstract
Background: Since drug resistance has become one of the predominant problems of health worldwide, it is necessary to use new methods to combat drug-resistant bacteria. In this regard, medicinal plants are considered one of the richest sources to produce antibiotics. The aim of this study was to investigate the antibacterial effects as well as total phenolic and flavonoid contents of a number of medicinal plants collected from the Chaharmahal and Bakhtiari provinces of India, in order to investigate their potential use for the production of new antibiotics.
Materials and Methods: In this experimental study, the maceration method was used to prepare hydroalcoholic extract of Medicago sativa, Onosma sericeum, Parietaria judaica L., Phlomis persica and Echinophora platyloba DC. The effect of these plants on Enterococcus faecalis (ATCC 29212) was investigated. To determine the antibacterial effect of the extracts, broth microdilution in sterile 96-well plate was used according to the McFarland standard (105 CFU/ml). The total phenolic content was assayed by using the Folin-Ciocalteu colorimetric method and expressed in terms of gallic acid equivalent. The total flavonoid content was assayed by aluminum chloride colorimetric method and expressed in terms of rutin equivalent.
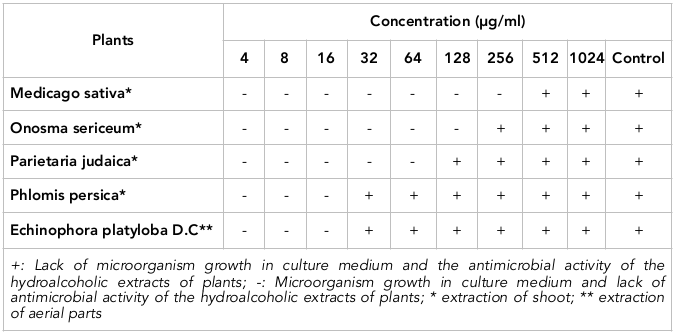
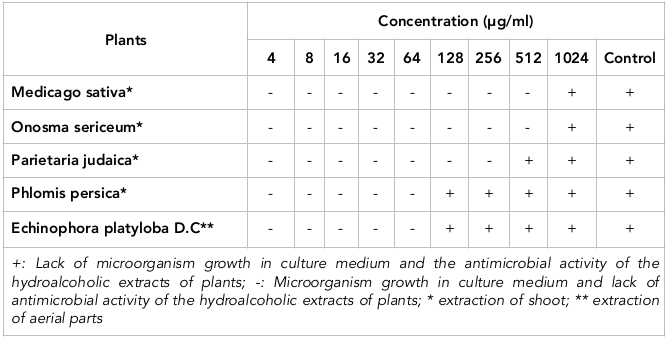
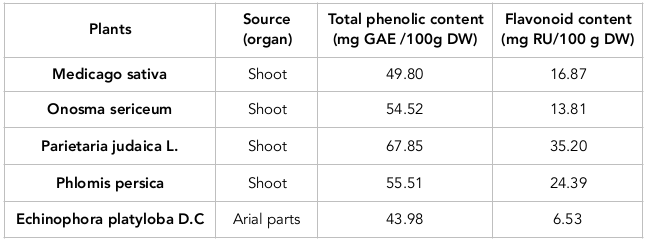
Results: Based on the results of this study, the 512, 256, 128, 32 and 32 µg/ml doses were determined to be the minimum inhibitory concentrations (MICs), and the 1024, 1024, 512, 128 and 128 μg/ml doses were derived as the minimum bactericidal concentration (MBCs) of M. sativa, O. Sericeum, P. judaica, P. persica and E. platyloba, respectively. E. faecalis and P. judaica contained the highest total phenolic content and flavonoid content, respectively.
Conclusion: Given the comparatively higher antibacterial effect of P. persica and E. platyloba, as well as the presence of phenolic and flavonoid compounds in these plants, it is recommended that these plants be further investigated in feasibility studies for the production of new antibiotics.
Introduction
Nowadays new bacterial resistance to commonly used chemical drugs has turned into a widespread phenomenon. Enterococcus faecalis is one of these bacteria. E. faecalis can be a cause of bacteremia, meningitis, septicemia, endocarditis, wound infection, infant infections and urinary tract infections Kafil and Asgharzadeh, 2014. In addition to vancomycin-resistant enterococci (VRE), this bacterium has been reported to acquire resistance to daptomycin, which has aroused concerns Werth et al., 2014.
With increasing drug resistance among bacteria, efforts are being made to seek out new therapies. Phytotherapy is one of the most promising therapies for many diseases Asadi-Samani et al., 2017Bahmani et al., 2016Kooti et al., 2016Moradi et al., 2017Rahimi-Madiseh et al., 2017Rahimifard et al., 2017Rouhi-Boroujeni et al., 2017Sarrafchi et al., 2016. Indeed, the collection and screening of medicinal plants can be helpful in areas with high potential for growth of medicinal plants Bahmani et al., 2014. Therefore, the present study was conducted to investigate the antibacterial effects of Medicago sativa, Onosma sericeum, Parietaria judaica L., Phlomis persica and Echinophora platyloba DC, and to determine their phenolic and flavonoid contents. These plants are grown in the Chaharmahal and Bakhtiari provinces of Iran, which are areas with high potential for the growth of medicinal plants. Understanding their antibacterial effects would address the suitable candidates for phytotherapy and the feasibility for production of new antibiotics.
M. sativa, also known as alfalfa and lucerne, comes from the Fabaceae family Sadowska et al., 2014. M. sativa is used as a food additive in the United States, Russia, North Africa and China because of their high vitamin content Shi et al., 2014. It produces secondary metabolites, such as coumarins, isoflavones, naphthoquinones, alkaloids and saponins, that have nematocidal, cytotoxic and antimicrobial effects Sadowska et al., 2014.
O. sericeum is a perennial plant that grows naturally in Iran and is a member of the Boraginaceae family Gharehmatrossian et al., 2016Naz et al., 2006. In addition to the roots of this herb (which is a dye and used in cosmetics), other properties have been reported for this plant, including antitumor, anti-inflammatory, antipregnancy, antimicrobial, cardiotonic and antiviral effects Gharehmatrossian et al., 2016.
P. judaica (Urticaceae) grows abundantly in urban areas of the Mediterranean region. P. judaica is a perennial herb, with individual plants consisting of many shoots emerging from a common rootstock Fotiou et al., 2011Mozaffarian, 2015. P. persica is from the family Lamiaceae. The genus Phlomis consists of 100 species worldwide, with 17 species in Iran. This herb in phytotherapy is used to treat topical wounds and respiratory diseases. Some other properties including analgesic, anti-diarrheatic, hemorrhagic ulcer-treating, antimalarial, anti-inflammatory, antimicrobial and immunosuppressive effects have also been reported for this plant Sarkhail et al., 2006. Moreover, some sources have reported tonic, diarrhea and free radical-inhibitory effects Hussain et al., 2010.
The Echinophora genus consists of 10 species of which Echinophora orientalis, Echinophora sibthorpiana, Echinophora cinerea and E. platyloba are present in Iran Shahneh et al., 2013. E. platyloba is used more often as a food additive in Iran Avijgan et al., 2010. This plant has been reported to exhibit antifungal, antioxidant and antibacterial properties Sharafati-chaleshtori et al., 2012.
Materials - Methods
Collecting plants
The plants were collected from different regions of the Chaharmahal and Bakhtiari provinces (such as Shahrekord, Teshniz, and Saman) between March 2016 and September 2016. The plants were identified as the plants of interest by a botanist (Dr. Shirmardi) at the Research Center of the Agricultural Jihad Organization of Chaharmahal and Bakhtiari province.
Extraction
Extraction was conducted by maceration of Medicago sativa, Onosma sericeum, Prietaria judaicaa L., Phlomis persica shoots, and from aerial parts of Echinophora platyloba D.C; these were done in triplicates (for 72h each time). In this method, water and butyric acid-free bitter ethanol at 30/70 ratio were used. The resulting extract was filtered using filter paper and evaporated under next-to-vacuum pressure and 40°C by a rotary evaporator to concentrate. The resulting solution was stored at -20°C until later use.
Preparing different dilutions of extract
The extracts were prepared using dimethyl sulfoxide (DMSO) and distilled water. Different dilutions (4, 8, 16, 32, 64, 128, 256, 512 and 1024 μg/ml) of the extracts were prepared using Mueller-Hinton broth agar. The maximum concentration of DMSO was 0.2% in the final concentration.
Preparing standard bacterial strains
Enterococcus faecalis (ATCC 29212) was purchased as lyophilized from Iranian Research Organization for Science and Technology.
Preparing microbial suspension
To prepare a microbial suspension equivalent to 0.5 McFarland standard (105 CFU/ml), a 24-hour culture of the bacteria was performed on blood agar, and then a suspension with 0.5 McFarland turbidity was prepared in normal saline.
Determining minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs)
The antibacterial effects of the extracts were determined by broth microdilution in a sterile 96-well plate with reference to 0.5 McFarland standard (105 CFU/ml). In this method, the first well was considered “culture medium + extract” (negative control), and the second well was considered “culture medium + bacterium” (positive control). After adding the culture medium at 95µl and the extracts at 100µl to microplate wells and diluting them, we incubated the samples at 37°C for 24h. The concentration of the last (most diluted) well without turbidity was considered MIC (Andrews, 2001). To determine MBC, we subsequently performed a culture of the samples of each tube at 10µl on Mueller-Hinton agar and left them at 37°C to incubate for 24h. The lowest concentrations of the extract in which the bacteria could not grow were considered MBCs. The tests were performed to determine the MICs and MBCs, and were conducted in triplicates (National Committee for Clinical Laboratory Standards, 2001).
Determining total phenolic and flavonoid content
Total phenolic content was measured by Folin-Ciocalteu colorimetric assay and expressed in terms of gallic acid equivalent. Total flavonoid content was measured by aluminium chloride colorimetric method and expressed in terms of rutin equivalent Dulf et al., 2016Karimi and Moradi, 2015Singleton et al., 1999.
Results
M. sativa extract could inhibit E. faecalis at 512 μg/ml. At 1024 μg/ml, M. sativa extract could destroy the bacteria. O. sericeum displayed more potent bacteriostatic activity against E. faecalis than M. sativa; the bactericidal activity of O. sericeum was the same as M. sativa. P. judaica showed MIC and MBC of 128 and 512 μg/ml, respectively. Moreover, 32 μg/ml of P. persica and E. platyloba inhibited E. faecalis, while 128 μg/ml of P. persica and E. platyloba eliminated E. faecalis.
The least potent bactericidal effects among the studied plants was seen by M. sativa and O. sericeum. Lastly, P. persica and E. platyloba showed the most potent antibacterial effects on E. faecalis ( Table 1 and Table 2 ).
According to Table 3 , all plants contained phenols and flavonoids. P. judaica had the highest total phenolic and flavonoid content. The plant with the least flavonoid content was E. platyloba ( Table 3 ).
Discussion
This present study was conducted to investigate the antibacterial effects as well as the total phenolic and flavonoid contents of a number of medicinal plants collected from the Chaharmahal and Bakhtiari provinces of Iran. The aim was to evaluate which plants could be considered as suitable alternatives for development of antibiotics to fight treatment-resistant bacteria.
Our findings showed the antibacterial effects of M. sativa on E. faecalis, with MIC of approximately 512 μg/ml and MBC of 1024 μg/ ml for this plant. Chavan et al. conducted a study to investigate the antibacterial properties of M. sativa. The methanol extract of this plant created inhibition zones of 23, 22 and 23 mm in diameter for Escherichia coli, Pseudomonas aeruginosa and Streptococcus aureus, respectively. The authors also reported an MIC of 37 μg/ml for E. coli, 12.03 mg/ml for P. aeruginosa and 111 μg/ml for S. aureus Chavan et al., 2015. The antibacterial effect of this plant could be due to their phenolic compounds. Notably, our study and the study of Chavan et al. demonstrate the optimal antibacterial effects of M. sativa.
Another plant investigated in our study was O. sericeum; its antimicrobial effect on E. faecalis was demonstrated- with MIC and MBC of 256 μg/ml and 1024 μg/ml, respectively. A study conducted by Naz et al., to examine the antimicrobial effects of another species from the Onosma genus (Onosma hispidum), showed that this plant could inhibit the growth of various bacteria and could have an antibacterial effect on E. faecalis Naz et al., 2006. The study by Vukic et al. on Onosma visianii showed that main components of this plant extract were 5,8-O-dimethyl isobutyrylshikonin, acetylshikonin, α-methylbutyrylshikonin, β-hydroxyisovalerylshikonin, isobutyrylshikonin, deoxyshikonin, and 5,8-O-dimethyl deoxyshikonin. Indeed, O. sericeum plant had a potent antibacterial effect not only on Enterococcus faecalis but also Bacillus megaterium, Micrococcus luteus, Staphylococcus epidermidis and Stenotrophomonas maltophilia Vukic et al., 2017. The results of the current study and other studies, thus, show that the species of the Onosma genus can have antibacterial effects on E. faecalis, which can be due to the presence of phenolic compounds in their aerial parts.
P. judaica, which was observed to have the highest phenolic and flavonoid content among the studied plants, also exhibited robust antibacterial effects on E. faecalis. Fares et al. who investigated the antibacterial effects of extracts of wild plants in Palestine showed that P. judaica had antibacterial effect on Streptococcus pneumoniae; the aqueous and ethanol extracts of this plant had an MIC of 3.125 and 100 mg/ml, respectively Fares et al., 2013. Our present study and other studies have demonstrated the antibacterial effects of this plant. The antibacterial effects of this species, therefore, is not an unexpected observation due to the presence of phenolic and flavonoid compounds. Moreover, it is not a surprising observation due to the fact that extracts from this plant can be isolated for its antibacterial compounds.
P. persica and E. platyloba in our study were found to exert the most potent antibacterial effect on E. faecalis when compared to the other studied plants. A phytochemical study showed the presence of relatively equal amounts of phenolic content in both plants. Amor et al. studied the antibacterial effects of Phlomis crinite. They reported that the essential oil of its flower had an inhibitory effect on S. aureus, E. faecalis and Salmonella typhimurium- with the MIC ranging between 39 μg/ml and 625 μg/ml Amor et al., 2008. Sarkhail et al. (2006) showed that P. persica shoot extract had chrysoeryol-7-O-β-D-glucoside, verbascoside and two other glycosidic flavonoids to which the antibacterial effects of this plant can be attributed Sarkhail et al., 2006. Besides that, Ranjbar and Babaei (2016) studied the antimicrobial properties of E. platyloba on different Salmonella species and concluded that this plant, at 150 mg/ml, exerted antifungal effect on Salmonella enteritidis and Salmonella typhi and, at 250 mg/ml, on Salmonella choleraesuis Ranjbar and Babaie, 2016. Entezari et al. (2009) investigated the antibacterial effects of E. platyloba, and reported that the methanol extract of this plant had an inhibitory effect on S. aureus and P. aeruginosa. Thus, at high doses, the E. platyloba plant could completely inhibit the growth of these bacteria. An investigation of the chemical compounds of the essential oil of E. platyloba also showed that monoterpenes and sesquiterpenes are the major compounds of this plant Rahimi-Nasrabadi et al., 2010. As it has been clearly shown in this study and other studies, E. platyloba is a plant that can be an appropriate choice to be further studied for feasibility as an antibacterial agent; it has potent antibacterial effects on different bacterial species as well as phenolic and flavonoid compounds.
Conclusion
All five plants were collected from the Chaharmahal and Bakhtiari provinces, had relatively equal amounts of phenolic compounds, and showed antibacterial effects on E. faecalis. It is recommended that these plants be further investigated in feasibility studies for the production of new antibiotics.
Abbreviations
DMSO: Dimethyl sulfoxide
DW: Dry weight
GAE: Gallic acid equivalent
MIC: Minimum inhibitory concentration
MBC: Minimum bactericidal concentration
RU: Rutin
Author Contribution
All authors equally contributed on all experiments; designed the study; wrote the manuscript; approved the manuscript for publication.
References
-
I. L. B.
Amor,
A.
Neffati,
M. B.
Sgaier,
W.
Bhouri,
J.
Boubaker,
I.
Skandrani.
Antimicrobial activity of essential oils isolated from Phlomis crinita Cav. ssp. mauritanica Munby. Journal of the American Oil Chemists’ Society.
2008;
85(9)
:
845-849
.
-
M.
Asadi-Samani,
N.
Bagheri,
M.
Rafieian-Kopaei,
H.
Shirzad.
Inhibition of Th1 and Th17 cells by medicinal plants and their derivatives: A systematic review. Phytotherapy Research.
2017;
31(8)
:
1128-1139
.
View Article PubMed Google Scholar -
M.
Avijgan,
M.
Mahboubi,
M.
Darabi,
M.
Saadat,
S.
Sarikhani,
N.
Kassaiyan.
Overview on Echinophora platyloba, a synergistic anti-fungal agent candidate. Journal of Yeast and Fungal research.
2010;
1(5)
:
88-94
.
-
M.
Bahmani,
A.
Sarrafchi,
H.
Shirzad,
M.
Rafieian-Kopaei.
Autism: Pathophysiology and promising herbal remedies. Current Pharmaceutical Design.
2016;
22(3)
:
277-285
.
View Article PubMed Google Scholar -
M.
Bahmani,
A.
Zargaran,
M.
Rafieian-Kopaei.
Identification of medicinal plants of Urmia for treatment of gastrointestinal disorders. Revista Brasileira de Farmacognosia.
2014;
24(4)
:
468-480
.
View Article Google Scholar -
S.S.
Chavan,
R.S.
Jadhav,
K.S.
Khemnar,
V.B.
Tambe.
Evaluation of antibacterial activity and phytochemical screening of Medicago sativa leaves. Int J Curr Res Acad Rev.
2015;
3(5)
:
308-313
.
-
F. V.
Dulf,
D. C.
Vodnar,
C.
Socaciu.
Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chemistry.
2016;
209
:
27-36
.
-
S.
Fares,
G.
Omar,
L.
Abdallah,
M.
Almasri,
A.
Slaileh,
Z.
Zurba.
Antibacterial activity of selected palestinian wild plant extracts against multidrug-resistant clinical isolate of streptococcus pneumonia. Journal of Pharmacy Research.
2013;
1(10)
:
963-969
.
-
C.
Fotiou,
A.
Damialis,
N.
Krigas,
J. M.
Halley,
D.
Vokou.
Parietaria judaica flowering phenology, pollen production, viability and atmospheric circulation, and expansive ability in the urban environment: Impacts of environmental factors. International Journal of Biometeorology.
2011;
55(1)
:
35-50
.
View Article PubMed Google Scholar -
S.
Gharehmatrossian,
Y.
Popov,
M.
Ghorbanli,
S.
Safaeian,
A.
Iranbakhsh.
Phytochemical and morphological evidences for shikonin production by plant cell cultures of Onosma sericeum Willd. Brazilian Archives of Biology and Technology.
2016;
59(0)
:
1-7
.
View Article Google Scholar -
J.
Hussain,
R.
Ullah,
F. U.
Khan,
Z.
Muhammad,
N.
Khan,
N. U.
Rehman.
Antiglycation and antimicrobial activities of the crude extract of Phlomis bracteosa. American-Eurasian Journal of Agricultural & Environmental Sciences.
2010;
7
:
634-636
.
-
H. S.
Kafil,
M.
Asgharzadeh.
Vancomycin-resistant enteroccus faecium and enterococcus faecalis isolated from education hospital of iran. Journal of Clinical Medicine.
2014;
9(4)
:
323-327
.
PubMed Google Scholar -
A.
Karimi,
M.T.
Moradi.
Total phenolic compounds and in vitro antioxidant potential of crude methanol extract and the correspond fractions of Quercusbrantii L. acorn. Journal of HerbMed Pharmacology.
2015;
4(1)
.
-
W.
Kooti,
Z.
Hasanzadeh-Noohi,
N.
Sharafi-Ahvazi,
M.
Asadi-Samani,
D.
Ashtary-Larky.
Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chinese Journal of Natural Medicines.
2016;
14(10)
:
732-745
.
View Article PubMed Google Scholar -
B.
Moradi,
S.
Heidari-Soureshjani,
M.
Asadi-Samani,
Q.
Yang,
A.
Saeedi-Boroujeni.
Efficacy and mechanisms of medicinal plants as immunotherapy in treatment of allergic rhinitis. Systematic Reviews.
2017;
8
:
1892-1899
.
-
V.
Mozaffarian.
Identification of medicinal and aromatic plants of Iran. Tehran: Farhang Moaser.
2015
.
-
S.
Naz,
S.
Ahmad,
S.
Ajaz Rasool,
S.
Asad Sayeed,
R.
Siddiqi.
Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiological Research.
2006;
161(1)
:
43-48
.
View Article PubMed Google Scholar -
M.
Rahimi-Madiseh,
P.
Karimian,
M.
Kafeshani,
M.
Rafieian-Kopaei.
The effects of ethanol extract of Berberis vulgaris fruit on histopathological changes and biochemical markers of the liver damage in diabetic rats. Iranian Journal of Basic Medical Sciences..
2017;
20(5)
:
552-556
.
PubMed Google Scholar -
M.
Rahimi-Nasrabadi,
M. B.
Gholivand,
M.
Niasari,
A.
Vatanara.
Chemical composition of the essential oil from aerial parts of Echinophora platyloba DC. from Iran. Faslnamah-i Giyahan-i Daruyi.
2010;
9
:
53-56
.
-
M.
Rahimifard,
F.
Sadeghi,
M.
Asadi-Samani,
K.
Nejati-Koshki.
Effect of quercetin on secretion and gene expression of leptin in breast cancer. Journal of Traditional Chinese Medicine.
2017;
37(3)
:
321-325
.
View Article Google Scholar -
R.
Ranjbar,
S.
Babaie.
Evaluation the antibacterial effects of Echinophora platyloba extracts against some Salmonella species. Electronic Physician.
2016;
8(2)
:
1943-1948
.
View Article PubMed Google Scholar -
H.
Rouhi-Boroujeni,
E.
Heidarian,
H.
Rouhi-Boroujeni,
F.
Deris,
M.
Rafieian-Kopaei.
Medicinal plants with multiple effects on cardiovascular diseases: A systematic review. Current Pharmaceutical Design.
2017;
23(7)
:
999-1015
.
View Article PubMed Google Scholar -
B.
Sadowska,
A.
Budzyńska,
M.
Więckowska-Szakiel,
M.
Paszkiewicz,
A.
Stochmal,
B.
Moniuszko-Szajwaj,
M. Różalska
Kowalczyk.
New pharmacological properties of Medicago sativa and Saponaria officinalis saponin-rich fractions addressed to Candida albicans. Journal of Medical Microbiology.
2014;
63(Pt 8)
:
1076-1086
.
View Article PubMed Google Scholar -
P.
Sarkhail,
H. R.
Monsef-Esfehani,
G.
Amin,
M. H. S.
Surmaghi,
A.
Shafiee.
Phytochemical study of Phlomis olivieri Benth. and Phlomis persica Boiss. Daru : Journal of Faculty of Pharmacy, Tehran University of Medical Sciences.
2006;
14
:
115-121
.
-
A.
Sarrafchi,
M.
Bahmani,
H.
Shirzad,
M.
Rafieian-Kopaei.
Oxidative stress and Parkinson’s disease: New hopes in treatment with herbal antioxidants. Current Pharmaceutical Design.
2016;
22(2)
:
238-246
.
View Article PubMed Google Scholar -
F. Z.
Shahneh,
S.
Valiyari,
A.
Azadmehr,
R.
Hajiaghaee,
S.
Yaripour,
A.
Bandehagh,
B.
Baradaran.
Inhibition of growth and induction of apoptosis in fibrosarcoma cell lines by Echinophora platyloba DC: In vitro analysis. Advances in Pharmacological Sciences.
2013;
2013
:
512931
.
View Article PubMed Google Scholar -
R.
Sharafati-chaleshtori,
M.
Rafieian-kopaei,
S.
Mortezaei,
A.
Sharafati-chaleshtori,
E.
Amini.
Antioxidant and antibacterial activity of the extracts of Echinophora platyloba D.C. African Journal of Pharmacy and Pharmacology.
2012;
6
:
2692-2695
.
-
Y.
Shi,
R.
Guo,
X.
Wang,
D.
Yuan,
S.
Zhang,
J.
Wang,
X. Wang
Yan.
The regulation of alfalfa saponin extract on key genes involved in hepatic cholesterol metabolism in hyperlipidemic rats. PLoS One.
2014;
9(2)
:
e88282
.
View Article PubMed Google Scholar -
V. L.
Singleton,
R.
Orthofer,
R. M.
Lamuela-Raventos.
Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin- Ciocalteu reagent. Methods in Enzymology.
1999;
299
:
152-178
.
View Article Google Scholar -
M.D.
Vukic,
N.L.
Vukovic,
G.T.
Djelic,
S.L.
Popovic,
M.M.
Zaric,
D.D.
Baskic,
G.B.
Krstic,
V.V. Kacaniova
Tesevic.
Antibacterial and cytotoxic activities of naphthoquinone pigments from Onosma visianii Clem. EXCLI Journal.
2017;
16
:
73-88
.
PubMed Google Scholar -
B.J.
Werth,
M.E.
Steed,
C.E.
Ireland,
T.T.
Tran,
P.
Nonejuie,
B.E.
Murray,
W.E.
Rose,
G.
Sakoulas,
J.
Pogliano,
C.A. Rybak
Arias.
Defining daptomycin resistance prevention exposures in vancomycin-resistant Enterococcus faecium and E. faecalis. Antimicrobial Agents and Chemotherapy.
2014;
58(9)
:
5253-5261
.
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 1 (2018)
Page No.: 1941-1951
Published on: 2018-01-23
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5588 times
- Download PDF downloaded - 1624 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress