Abstract
Background: Thyroid cancers are common endocrine tumors with diverse medical and histological structures. During development/progression from normal to neoplastic cell, there is a gradual increase in the function/activity of proto-oncogenes, transcription factors and metastasis elements. The main objective of this study is to evaluate per-oxidation of lipid content, total oxidative stress, and the profile of homocysteine (and DNA damage) in the erythrocytes of thyroid carcinoma patients as compared with those of control subjects.
Methods: All risk variables and biochemical analyses were quantitatively determined using standard methods.
Results: A noteworthy increase in malondialdehyde, globulin, and DNA damage in thyroid carcinoma patients were repeatedly observed. In contrast, healthy individuals showed an increased level of HDL-C and total anti-oxidant response.
Conclusion: It is suggested that these parameters have a pivotal role in the diagnostic process of determining thyroid carcinoma patients. Oxidized products of macromolecules in the blood of such patients impart major function in causing thyroid carcinoma disease.
Introduction
Among all malignant cancers, thyroid carcinoma is being witnessed in increasing cases in European countries, USA and Canada Siti et al., 2015Mancini et al., 2016Valko et al., 2007. It is more aggressive and common in the 30-60 year age group. Human body cells consume oxygen to generate energy but oxygen is inherently fatal for their survival Siti et al., 2015 as it causes oxidative stress (OS), which is due to the unbalanced level of generation of pro-oxidant molecules and production of anti-oxidant defenses the body synthesizes Mancini et al., 2016. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are considered as both toxic and beneficial species Valko et al., 2007. If in high concentration, they react with DNA, proteins and lipids, and can chemically alter their function Le Bras et al., 2005. In cancer cells, oxidative stress has been linked to the regulation of numerous cellular processes including DNA damage, cellular adhesion and migration, proliferation, and the regulation of cell survival or death Mancini et al., 2016. Earlier studies on the role of ROS and RNS in tumor development revealed that they both act as DNA-damaging agents by increasing the mutation rate within cells alarmingly and enhancing oncogenic transformation Jackson and Loeb, 2001.
ROS and RNS have the inherent potential to enhance specific intracellular events during communication of messages between cellular parts. As well, they take part in the development of tumors and their spreading to other parts of the body by regulating related processes including proliferation, death and mortality. These effects are due to hydroxyl and peroxynitrite radicals which possess the capacity to act in a non-specific and destructive manner. They can specifically activate certain intracellular signaling cascades and, thus, contribute to tumor development and metastasis through the regulation of cellular phenotypes as mentioned above Karbownik and Lewinski, 2003. According to the suggestions of growing evidences, the production of ROS is highly controlled in many cases, acting on specific downstream targets Finkel, 2003.
Oxidative stress is very common in thyroid tissues while consuming hydrogen peroxide (H2O2) for thyroxin synthesis. Inflammation and oxidative stress are closely linked processes Mancini et al., 2016Fuejita, 2002. OS causes hormonal derangement in a reciprocal way Mancini et al., 2016. OS is also linked with hyperthyroidism and hypothyroidism in all animals. However, very little is known about the oxidant/antioxidant balance in thyroid cancer (Sadani, 1996). Papillary thyroid cancer comprises of 57-89% of all thyroid malignancies as reported by previous reports from this region Abdulmughni et al., 2004Mulaudzi et al., 2001. In this region of the world, the ratio of female to male is 2.5 to 4:1, and is comparable to international findings. Yet, there is no study to date evaluating the effects of complete resection of the thyroid tissue on this balance.
Serum malondialdehyde (MDA) is frequently utilized as an indicator for oxidative damage in tissues and cells Finkel, 2003Erel, 2005. In cells, the peroxide level is determined by the balance between the production and elimination of lipid peroxides. The disturbance in this balance can be caused by decrease in cellular defenses and/or by the remarkable increase in peroxidative reactions Karbownik and Lewinski, 2003.
Thyroid glands are surgically removed as an initial therapy for the treatment. The purpose of this surgery is to eliminate the tissues which have been affected by the tumor. Iodine-131 is given post-operatively to destroy any remnants left after surgery. It may be effective in doing the whole body scan (WBS) for the persistence of cancer and for decreasing the long-term effects of recurrent carcinoma.
Thus, in order to further delineate the role of oxidant/antioxidant balance in the thyroid cancer, the aim of this study was to analyze the lipid peroxidation outcome (malondialdehyde) in blood samples of thyroid cancer patients after the thyroidectomy operation, and compared with healthy controls.
Materials - Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). All kits were procured from the local suppliers representing the multi-national chemical companies, as indicated in the text.
Subjects
Thirty subjects diagnosed with thyroid carcinoma were included in this study and advised to undergo thyroidectomy at the Punjab Institute of Nuclear Medicines (PINUM) in Faisalabad, Pakistan. Thirty healthy subjects (age-, genetic background-, and sex-matched) related to the patients were considered as control subjects. Informed consent was obtained from all the subjects in this study. This work was performed according to standard ethical guidelines of local authorities.
Sample Collection
A total of 60 blood samples were collected aseptically from patients (n=30) and controls (n=30) into 4 ml vacutainers with and without K2-EDTA from the antecubital vein of each participant diagnosed for thyroid cancer. Blood samples were processed for plasma and serum. Analysis of all samples was performed in triplicate for different variables in GCUF laboratory. A physician regularly conducted the physical examination of all participants on this study. Blood pressure of all participants was measured by using sphygmomanometer and readings were recorded as systolic and diastolic pressure. The range of body mass index (BMI) was 24.83 ± 0.53 for control subjects; thyroid carcinoma patients had BMI range of 26.14 ±2.53, which was not significantly different.
Serum Biochemistry
Hemoglobin concentration (Hb; g/dL) was determined by cyanmethemoglobin method. The absorbance was measured at 540 nm on a spectrophotometer BENJAMIN, 1978 Commercially available Fluitest Glu Biocon kit (Lot# H265) was used for testing glucose levels via a spectrophotometer (Screen Master #35510). Glycosylated hemoglobin (HB; HbA1c; %) was determined using the boronate affinity chromatography for GlycosalTM test to assess the quantity of glycosylated hemoglobin fragments from that of non-glycosylated fragments. The % HbA1c for the samples was obtained from an algorithm measuring both fragments.
The erythrocyte sedimentation rate (ESR; mm/h) was determined by Westergren method Benjamin, 1978. Total homocysteine (tHcy) was determined by the application of homocysteine microtiter plate assay in sera of normal subjects and of patients. Total oxidant status (TOS; µmol H2O2 equiv/L) was measured using an automated colorimetric method Karbownik and Lewinski, 2003Finkel, 2003. Total antioxidant response (TAR) or total antioxidant status (TAS; mmol trolox Equiv./L) was determined using an automated calorimetric method Erel, 2005. Malondialdehyde (MDA; µmol/L) was determined using laboratory kit and spectrophotometer (UV/VIS) Fujita, 2002.
DNA damage was measured using 8-hydroxy-deoxy guanosine enzyme immunoassay (EIA) kit as described earlier Jackson and Loeb, 2001. Triiodothyronine (T3; ng/ml) was determined using T3 EIA kit (BioCheck Inc., South San Francisco, CA, USA; cat. # BC-1005). For the measurement of total thyroxin (T4) concentration, EIA kit (BioCheck) was used. Total thyroid stimulating hormone (TSH) was assessed using EIA kit (BioCheck) as previously done Fujita, 2002. Estrogen (estradiol, E2) was determined by using EIA kit as well Sadani, 1996. Aspartate aminotransferase (AST) and alanine aminotransaminase (ALT) were determined by Reitman–Frankel colorimetric method by using a commercial kit (Crescent Diagnostics; cat. #CZ902C). Triiodothyronine was quantitatively determined by the T3 EIA kit (BioCheck). The overall T4, TSH and E2 concentrations were quantified, as aforementioned, by their respective EIA kits (BioCheck).
Statistical Analysis
From the data, mean ± SD were calculated. To calculate the differences between normal (control subjects) and thyroid carcinoma patients, data were analyzed by three-way analysis of variance Steel, 1997. In case of significant differences, Duncan Multiple Range Test (DMRT) was applied Duncan, 1955. GraphPad Prism Software (San Diego, CA, USA) was used to calculate statistical significance of the results.
Results
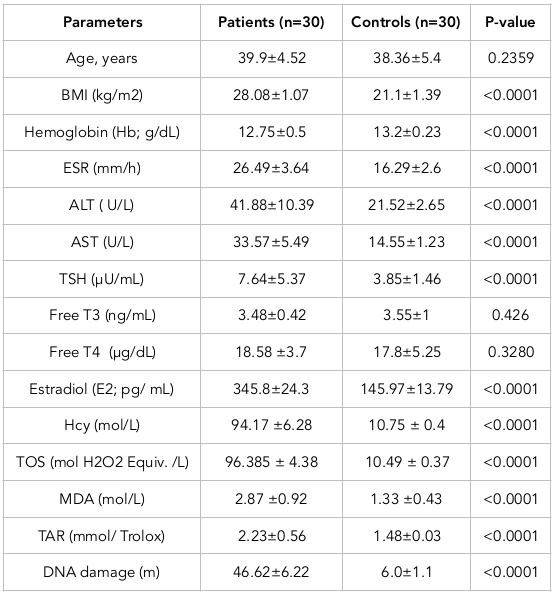
Mean ± SD of the age (in years) of the patients (39.9±4.52) and control subjects (38.36±5.4) were not significantly different. The median age at the time of diagnosis was 45 to 50 years.
Anthropometry
Analysis of various anthropometric parameters of controls and thyroid cancer patients was done. One-way analysis of variance (ANOVA) calculation suggested that BMI was significantly higher (P0.0008) in patients compared to that of normal subjects. Hemoglobin was not significantly (P=0.3891) different between diseased and normal subjects, i.e. Hb levels were within normal range for both. However, in the case of ESR, it was significantly higher (P=0.0188) in diseased cases than in normal subjects ( Table 1 ).
Serum Biochemical Parameters
All risk factors of thyroid carcinoma were significantly higher in diseased subjects. In contrast, glycosylated Hb was non-significantly (p=0.0914) higher in patients. Diseased subjects had relatively higher values of ALT, AST and TSH (p0.0001) than those of normal subjects. T4 and T3 were considered not significantly different in subjects versus controls (p=0.426 and p=0.3280, respectively, for T4 and T3). Diseased subjects had showed significantly higher values of estradiol, homocysteine and TOS (p0.0001) than those of their respective normal subjects.
Diseased subjects had a significantly higher value of MDA (p=0.0001) than those of control subjects. DNA damage was significantly (p0.0001) higher in diseased than normal subjects. Moreover, TAR was considered significantly (p0.0001) higher in healthy individuals as compared to that for diseased subjects.
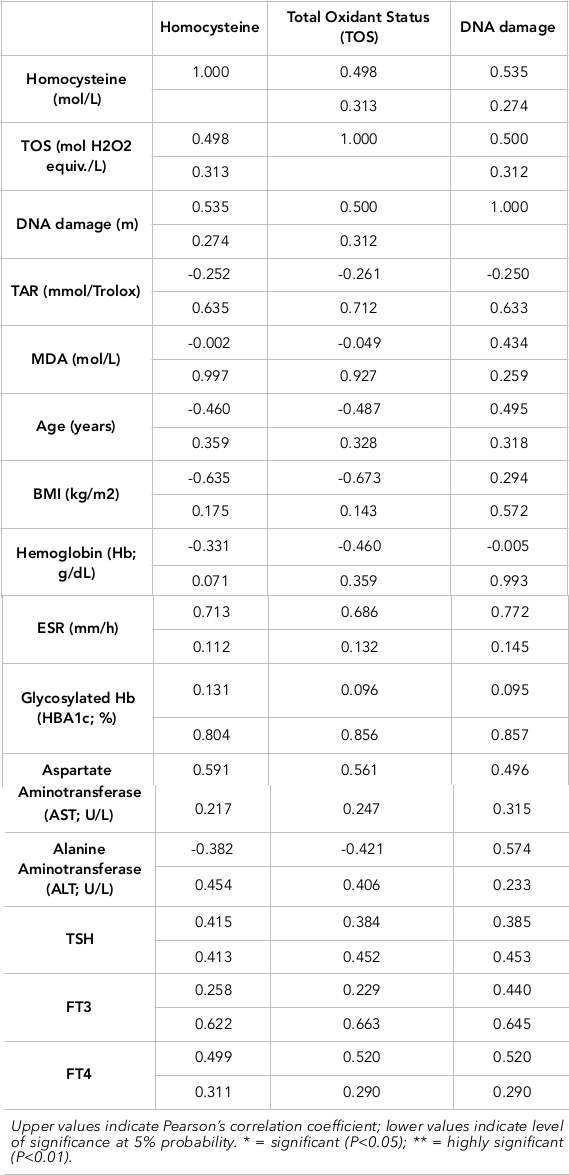
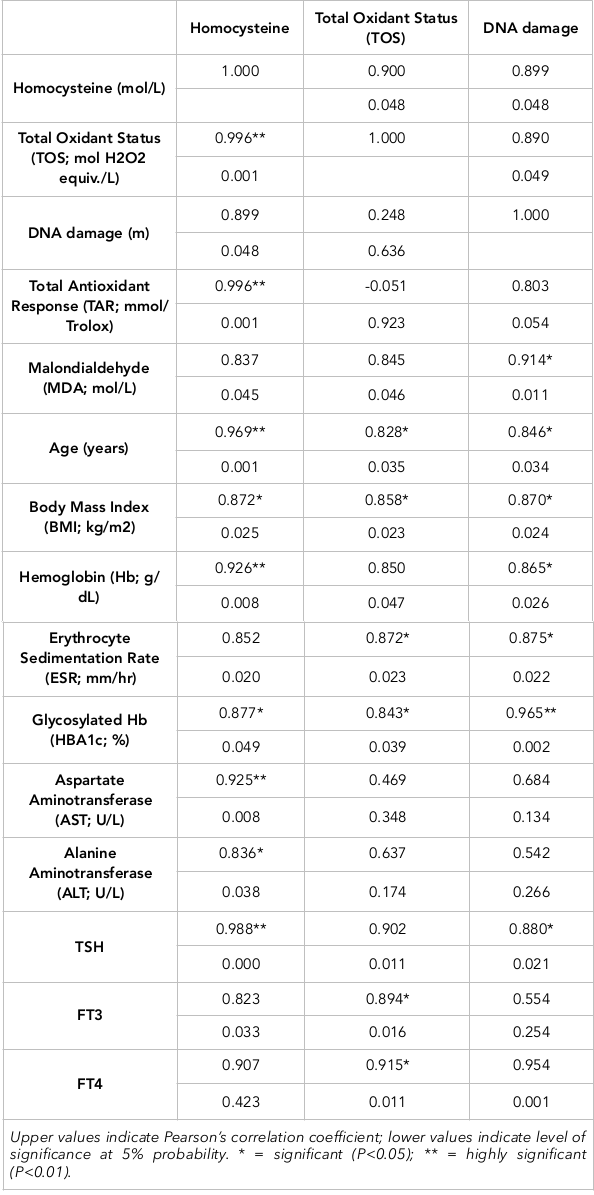
The Pearson correlation of homocysteine, TOS and DNA damage with anthropometric measurements, biochemicals, lipid profile, LFTs, serum hormones and health biomarkers have been evaluated for control subjects and diseased subjects. Serum homocysteine showed a negative correlation with Hb, albumin, cholesterol, triglycerides, HDL, LDLs, TSH, and estrogen in diseased subjects. A positive but non-significantly different relationship of homocysteine was established with BMI, RBS, HbA1C, T3, T4, TOS, TAR, MDA and DNA damage. It was related significantly with ESR, ALT, AST, TOS and DNA damage but negatively with TAR. The Pearson correlation of DNA damage with anthropometrics, biochemicals, lipid profile, LFTs, serum hormones and health biomarkers of patients are presented in Table 3. DNA damage showed a negative correlation with ESR, HBA1C, cholesterol, TGs, LDL, T3, T4 and E2. A positive relationship of DNA damage was established with BMI, hemoglobin, random blood sugar, albumin, total proteins, ALT, AST, TSH, TOS, homocysteine, MDA and TAR.
The Pearson correlation of TOS with anthropometrics, biochemicals, lipid profile, LFTs, serum hormones and health biomarkers in healthy control subjects are also shown. TOS showed a negative correlation with hemoglobin, HBA1C, triglycerides, HDL and T3. A positive relationship of TOS was established with BMI, ESR, random blood sugars, total proteins, albumin, cholesterol, LDL, ALT, AST, TSH, T4, estrogen, homocysteine, MDA and DNA damage. Overall, statistical analyses showed a positive relationship of thyroid carcinoma with DNA damage, oxidative stress, MDA and homocysteine level; indeed, these all are potential markers of thyroid carcinoma ( Table 2 Table 3 ).
Discussion
The demographic characteristics of thyroid cancer patients are comparable with those of European countries. Even so, the clinical reports are quite diverse and more aggressive. Age and sex were not correlated with aggressiveness of disease. Of the differentiating thyroid cancers, PTC (papillary thyroid cancer) was mostly observed (76.66%), whereas FTC (follicular thyroid cancer) was less frequently observed (13.33%) as was Hurthle cell cancer (10% of total patients).
We observed very few children and adolescents affected with PTC and FTC (2 patients in our study); regarding age onset the chances of PTC and FTC increased in adults. The median age of diagnosis (based on our criteria) in this study was 45 to 50 years. We observed a higher ratio of thyroid carcinoma in women as compared to men (5 women for every 2 men).
Our study demonstrated that BMI was significantly high in thyroid carcinoma patients compared to controls (p=0.0008). In such patients, thyroid glands were removed surgically and patients were given thyroxin (orally) so that their body metabolism would function like normal individuals Kitahara et al., 2011. Kitahara et al. suggested in their work that for both men and women, BMI was found to be linked with thyroid carcinoma risk. Their findings give a strong indication that obesity is an independent risk factor for thyroid cancer.
In our study, hemoglobin was found to be within normal range in thyroid carcinoma patients. It was not significantly different in cancer patients versus healthy controls. The same was true for ESR; it was not significantly different between cancer patients and control subjects. However, ESR was slight above normal range in patients who were Hepatitis C Virus (HCV) positive.
ALT and AST are linked with liver function; their concentrations can be used to detect hepato-cellular injury and reflect the status of the liver. In the present study, ALT and AST levels were higher in several cancerous patients. Both AST and ALT concentrations are elevated in many hepatic diseases. However, in differentiating cholestatic injury from hepatocellular injury of the liver, aminotransferases are considered useful. Damage in erythrocytes, pancreas, heart and kidney cells are related to activity of AST.
In euthyroid patients, the thyroid gland is removed (thyroidectomy) but the thyroid hormones remain normal from administration of drugs to the patients. T4 and T3 can be measured as free thyroxine (FT4) and free triiodothyronine (FT3), which are indicators of thyroxine and triiodothyronine activities in the body. They can also be measured as total thyroxine and total triiodothyronine, which also depend on the thyroxine and triiodothyronine that are bound to thyroxine-binding globulin. A related parameter is the free thyroxine index, which is total thyroxine multiplied by thyroid hormone uptake which, in turn, is a measure of the unbound thyroxine-binding globulins. Normal levels of thyroid hormones are achieved by giving thyroxine according to the thyroid function test (TFT) levels, which control the normal body mechanism. In this study, we followed the trend and values of TSH, T3, and T4; they were considered not significant as they were in normal ranges.
Thyroxine therapy is withdrawn sometimes in patients to perform radioactive whole body scan (WBS). This allows checking if any remnants of cancerous tissue are left; if so, they are eliminated by radioactive iodine (I131). Moreover, Rosen and his colleagues proposed that risk of osteoporosis and accelerated bone loss are higher in those patients who take suppressive doses of T4 Rosen, 1998. In this case study, many self-complaints of patients were noted that were related to cramps and pain in body, especially legs. Serum calcium and vitamin D levels were found to be abnormal in those patients.
Estrogen was significantly higher than that in diseased males or control subjects. It is assumed that females have a higher ratio of thyroid tumors than males. Epidemiological studies have proposed that the pathogenesis of thyroid tumors may be associated with the use of estrogens. Manole and his group proposed that females had a 3-fold higher incidence of thyroid tumours than that of males Manole et al., 2001. Expression levels of estrogen receptor α in males and females are almost same, but in response to 17β-estradiol, there is a significant increase in estrogen receptor α expression levels. 17β-estradiol stimulates benign and malignant thyroid cells which can result in enhanced expression of cyclin D1 protein and increased cellular proliferation, which play vital roles in the regulation of the G1/S transition of the cell cycle.
Health biomarkers are a good source for detecting any changes or damage occurring in body. This case study included five health biomarkers, namely Homocysteine, Total Oxidant Status (TOS), Malondialdehyde (MDA), Total Antioxidant Response (TAR) and DNA damage. These markers may provide insight into whether thyroid cancer is associated with an oxidant/antioxidant imbalance. A positive relationship of TOS was found with random blood sugars, total proteins, albumin, cholesterol, LDL, TSH, T4, estrogen and MDA.
Homocysteine is significantly higher (p0.0001) in hypothyroidism conditions of patients. During short term iatrogenic hypothyroidism, Lien et al. found a significant increase in both plasma total homocysteine (tHcy) and serum cholesterol, as well as changes in renal function which are probably explained by the tHcy response Lien et al., 2000. Moreover, in hypothyroid patients the risk of cardiovascular diseases is increased by the increase of both plasma tHcy and serum cholesterol. In the present study, a positive relationship of homocysteine was established with hemoglobin, erythrocyte sedimentation rate, glycosylated hemoglobin, ALT, FT3, FT4, and DNA damage. In thyroid cancer patients, it was observed that TOS and DNA damage were significantly higher than normal hormone levels.
In the thyroid, pathological and physiological processes in the glands are associated with reactive ROS and free radicals. Oxidative stress can be triggered by imbalance between the production and degradation of ROS, which can lead to damage of different cell components, such as DNA, lipids and proteins. During utilization of H2O2 in thyroid tissues, oxidative stress is common for thyroxin synthesis, ROS production by inflammation, and when tumor cells undergo active proliferation FUJITA, 2002.
Our results show that BMI, hemoglobin, random blood sugar, albumin, triglycerides, HDL, LDL, ALT, AST, TSH, homocysteine, MDA and TOS have a positive correlation with DNA damage. From the evidence in literature, the activity of specific oncogenes and the development of neoplastic transformation (by in vivo and in vitro means) involves free radicals in the initiation mechanism. Many published studies have stated that agents that have the ability to remove free radicals or interfere with the activities of free radicals can also have the potential to trigger downstream neoplastic mechanisms at the cellular level as well as molecular level Sadani, G.R., 1996.
In our study, MDA were non-significantly high in all patients. Studies in the literature explain that in post thyroidectomy, serum MDA levels significantly decrease compared to prethyroidectomy levels but are still significantly higher than the control group’s level Akinci et al., 2008. Systemic oxidant/antioxidant status can be better understood or indicated by various levels of MDA or serum enzyme activities. However, these levels cannot indicate real changes occurring in the thyroid directly because many factors are involved to modify serum outcome.
Total antioxidant response (TAR) is also not very significant in this case study. This is because there was not much increase in lipid per-oxidation and damage to the antioxidant defense system. In the prevention of cancer and oxidative stress-related diseases, there are many natural antioxidant agents that have been found to be involved; these include vitamin A (retinoids), carotenoids, selenium, vitamin E (tocopherols), and vitamin C (ascorbic acid). β-carotene and retinoids have the ability to act as anticarcinogentic agents by antagonizing the biological effects of pro-oxidants on protein kinase C Carter and Kane, 2004.
Nevertheless, DNA damage and apoptotic responses are not found linked with p73 (protein of thyroid cancer cells). Notably, cell cycle can be arrested by overexpression of p73 in thyroid cancer cells but p73 is unable to induce cell death. Loss of biological function of P73 in neoplastic thyroid cells can be explained by its interaction with mutant p53 and variants of p73 (DeltaNp73)Taccaliti and Boscaro, 2009. It has been suggested that the development of thyroid malignancies may involve functionally disturbed p73 which may serve as a therapeutic target for thyroid cancer Frasca et al., 2003. Other oncogenes are Ras proteins, Braf proteins, Trk, RET/PTC, Galectin3, RREB1, TPO, PPFP and β-catenin; if mutations occur in these oncogenes it could lead to thyroid carcinoma. As indicated in the pathway, another tumor suppressor gene is p53 (a nuclear protein); if mutated, it could lead to thyroid cancer. The increased incidence of poorly differentiated and anaplastic carcinomas, but not well-differentiated tumors, seem to be related to mutations in p53 and β-catenin; this may suggest direct molecular triggering of tumor dedifferentiation Nikiforov, 2004.
Conclusion
Among endocrine malignancies, thyroid cancer is perhaps the most prevalent. Indeed, thyroid cancer has a high incidence and is the predominant endocrine cancer. The results of the present study suggest there is a notable increase in MDA and DNA damage in thyroid carcinoma patients. In contrast, healthy individuals showed increased levels of HDL-C and TAS. It is suggested that these parameters play pivotal roles in the diagnostic process of determining thyroid carcinoma in patients. Oxidized products of macromolecules in the blood of such patients impart major function in causing thyroid carcinoma disease.
Abbreviations
ALT: Alanine aminotransaminase
ANOVA: Analysis of variance
AST: Aspartate aminotransferase
BMI: Body Mass Index
MDA: Melanodialdehyde
OS: Oxidative stress
RNS: Reactive nitrogen species
ROS: Reactive oxygen species
TAR: Total antioxidant response
tHcy: Total homocysteine
WBS: Whole body scan
Author Contribution
MU SB performed the experimental work and collect the data. MIR supervised and designed the study and contributed to the analysis of the data, AH, ZI, FH, SF, QAA, edited the first draft. LK contributed to the analysis and interpretation of the data revised the manuscript. All authors reviewed and commented the final draft.
References
-
Y. A.
Abdulmughni,
M. A.
Al-Hureibi,
K. A.
Al-Hureibi,
M. A.
Ghafoor,
A. H.
Al-Wadan,
Y. A.
Al-Hureibi.
Thyroid cancer in Yemen. Saudi Medical Journal.
2004;
25(1)
:
55-59
.
PubMed Google Scholar -
M.
Akinci,
F.
Kosova,
B.
Çetin,
A.
Sepici,
N.
Altan,
S.
Aslan,
A.
Çetin.
Oxidant/antioxidant balance in patients with thyroid cancer. Acta Cirurgica Brasileira.
2008;
23(6)
:
551-554
.
View Article PubMed Google Scholar -
M. M.
BENJAMIN.
Outline of veterinary clinical pathology. Ames, Iowa State University Press.
1978
.
-
C. A.
Carter,
C. J.
Kane.
Therapeutic potential of natural compounds that regulate the activity of protein kinase C. Current Medicinal Chemistry.
2004;
11(21)
:
2883-2902
.
View Article PubMed Google Scholar -
D. B.
Duncan.
Multiple Range and Multiple F Tests. Biometrics.
1955;
11(1)
:
1
.
View Article Google Scholar -
O.
Erel.
A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical Biochemistry.
2004;
37(2)
:
112-119
.
View Article PubMed Google Scholar -
O.
Erel.
A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry.
2005;
38(12)
:
1103-1111
.
View Article PubMed Google Scholar -
T.
Finkel.
Oxidant signals and oxidative stress. Current Opinion in Cell Biology.
2003;
15(2)
:
247-254
.
View Article PubMed Google Scholar -
F.
Frasca,
V.
Vella,
A.
Aloisi,
A.
Mandarino,
E.
Mazzon,
R.
Vigneri,
P.
Vigneri.
p73 tumor-suppressor activity is impaired in human thyroid cancer. Cancer Research.
2003;
63(18)
:
5829-5837
.
PubMed Google Scholar -
T.
Fujita.
[Formation and removal of reactive oxygen species, lipid peroxides and free radicals, and their biological effects]. Yakugaku Zasshi.
2002;
122(3)
:
203-218
.
View Article PubMed Google Scholar -
A. L.
Jackson,
L. A.
Loeb.
The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutation Research. Fundamental and Molecular Mechanisms of Mutagenesis.
2001;
477(1-2)
:
7-21
.
-
M.
Karbownik,
A.
Lewinski.
The role of oxidative stress in physiological and pathological processes in the thyroid gland; possible involvement in pineal-thyroid interactions. Neuroendocrinology Letters.
2003;
24(5)
:
293-303
.
PubMed Google Scholar -
C. M.
Kitahara,
E. A.
Platz,
L. E. B.
Freeman,
A. W.
Hsing,
M. S.
Linet,
Y.
Park,
A.
Berrington de González.
Obesity and thyroid cancer risk among U.S. men and women: A pooled analysis of five prospective studies. Cancer Epidemiology, Biomarkers Prevention.
2011;
20(3)
:
464-472
.
-
M.
Le Bras,
M. V.
Clément,
S.
Pervaiz,
C.
Brenner.
Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histology and Histopathology.
2005;
20(1)
:
205-219
.
PubMed Google Scholar -
E. A.
Lien,
B. G.
Nedrebø,
J. E.
Varhaug,
O.
Nygård,
A.
Aakvaag,
P. M.
Ueland.
Plasma total homocysteine levels during short-term iatrogenic hypothyroidism. The Journal of Clinical Endocrinology and Metabolism.
2000;
85(3)
:
1049-1053
.
View Article PubMed Google Scholar -
A.
Mancini,
C. Di
Segni,
S.
Raimondo,
G.
Olivieri,
A.
Silvestrini,
E.
Meucci,
D.
Currò.
‘Thyroid Hormones, Oxidative Stress, and Inflammation’. 2016
.
-
D.
Manole,
B.
Schildknecht,
B.
Gosnell,
E.
Adams,
M.
Derwahl.
Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. The Journal of Clinical Endocrinology and Metabolism.
2001;
86(3)
:
1072-1077
.
View Article PubMed Google Scholar -
T. V.
Mulaudzi,
P. K.
Ramdial,
T. E.
Madiba,
R. A.
Callaghan.
Thyroid carcinoma at King Edward VIII Hospital, Durban, South Africa. East African Medical Journal.
2001;
78(5)
:
242-245
.
View Article PubMed Google Scholar -
Y. E.
Nikiforov.
Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocrine Pathology.
2004;
15(4)
:
319-327
.
View Article PubMed Google Scholar -
H. N.
Rosen,
A. C.
Moses,
J.
Garber,
D. S.
Ross,
S. L.
Lee,
L.
Ferguson,
S. L.
Greenspan.
Randomized trial of pamidronate in patients with thyroid cancer: Bone density is not reduced by suppressive doses of thyroxine, but is increased by cyclic intravenous pamidronate. The Journal of Clinical Endocrinology and Metabolism.
1998;
83(7)
:
2324-2330
.
View Article PubMed Google Scholar -
G. R.
Sadani,
G. D.
Nadkarni.
Role of tissue antioxidant defence in thyroid cancers. Cancer Letters.
1996;
109(1-2)
:
231-235
.
View Article PubMed Google Scholar -
H. N.
Siti,
Y.
Kamisah,
J.
Kamsiah.
The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascular Pharmacology.
2015;
71
:
40-56
.
View Article PubMed Google Scholar -
Robert GD
Steel,
H.
James,
David A. Dickey
Torrie.
Principles and procedures of statistics: A biological approach. New York : McGraw-Hill, cop.
1997
.
-
A
Taccaliti,
M.
Boscaro.
Genetic mutations in thyroid carcinoma. Minerva Endocrinol.
2009;
34
:
11-28
.
-
M.
Valko,
D.
Leibfritz,
J.
Moncol,
M. T.
Cronin,
M.
Mazur,
J.
Telser.
Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry Cell Biology.
2007;
39(1)
:
44-84
.
View Article PubMed Google Scholar -
S. B.
Weg.
Spectrophotometer simpler to use and higher in dependability thanks to mounting of a large-size color LCD. no date
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 1 (2018)
Page No.: 1952-1966
Published on: 2018-01-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4920 times
- Download PDF downloaded - 1228 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress