Abstract
Background: Chronic Kidney Disease (CKD) is progressive kidney damage in which the glomerular filtration rate (GFR) decreases by 15% of that performed by normal kidneys, with the result that the kidneys are no longer to function effectively and this condition is treated with either kidney transplantation or dialysis. Hemodialysis (HD) is a process in which the blood passes through a machine (dialyzer) to remove waste. Angiotensin Converting Enzyme (ACE) is one of the major components of the renin-angiotensin aldosterone system (RAAS) that is responsible for blood pressure regulation. Insertion/Deletion I/D polymorphism of a 287 bp Alu repeat sequence in introns 16 of the ACE gene results in three genotypes I/D, D/D and I/I. The aims of this study were to evaluate ACE genotypes and allele frequency among HD patients and control subjects and to examine the association between ACE genotypes with HD risk.
Methods: A retrospective case-control study included 186 subjects, 86 HD patients and 100 healthy individuals as a control. EDTA whole blood samples were collected from all participants for DNA extraction, and the ACE gene polymorphism was analyzed by using the Polymerase Chain Reaction (PCR) technique. Additionally, all individuals were requested to complete the questionnaire.
Results: The initial results demonstrated that the D/D genotype was the most common genotype in all subjects and the association between ACE genotypes with HD occurrence was not statistically significant in the Gaza Strip-Palestine. Furthermore, no statistically significant association was detected between the polymorphism and the study groups (p-value = 0.511).
Background
Chronic Kidney Disease (CKD) is a condition in which there is progressive and irreversible kidney damage for three months or more caused by abnormalities in the structure or the function of the kidneys, as well as a decrease in the glomerular filtration rate (GFR). Abnormal parameters in the blood (serum creatinine) and urine tests (urine albumin), pathological markers or imaging tests for kidneys imply kidney dysfunction [1]. GFR is considered to be the best estimate of kidney function and determines the stage of kidney disease; if the GFR is less than 15% than that performed by normal kidneys and the kidneys are no longer capable of functioning normally, renal failure occurs. For urine albumin, accurate quantification of the amount of albumin lost in the urine has important clinical connotations: healthy adults excrete less than 30 mg of albumin in 24 hours; excretion of amounts between 30 and 300 mg in 24 hours is termed microalbuminuria, and excretion of amounts in excess of 300 mg in 24 hours is termed macroalbuminuria [2]. All these conditions are treated with dialysis or kidney transplantation. Hemodialysis (HD) is the process in which the blood is passed through a machine (dialyzer) to remove waste like urea, restore electrolyte balance and remove the extra fluid and this process is usually performed at a hospital or dialysis center [3].
End-stage renal disease (ESRD) occurs when patients who are undergoing dialysis therapy have increased morbidity and mortality rates compared with the general population, and about 40% of deaths in HD are related to cardiovascular causes [4]. There are many risk factors for cardiovascular disease (CVD) in patients treated with dialyses, such as diabetes, hypertension (HTN), inflammation, anemia, oxidative stress, vascular calcification and changes in the Renin Angiotensin Aldosterone System (RAAS) [5].
Recently, the genetic variants in RAAS and its association with the components of metabolic syndrome have gained importance. The Angiotensin Converting Enzyme (ACE) gene is one of the most studied genes that produce a protein that acts on Angiotensin I (AngI) to produce Angiotensin II (AngII), which is a potent vasoconstrictor in RAAS that regulates blood pressure and extracellular volume [6].
ACE gene is mapped on chromosome 17q23.3 encoded by a 21 Kb gene that consists of 26 exons and 25 introns; more than 160 polymorphisms in the ACE gene are listed in NCBI records, and the majority are single nucleotide polymorphisms. In total, 34 of the polymorphisms occur in coding regions, and 18 of them are missense mutations. The ACE gene encodes two isoforms: one is expressed in somatic tissue called somatic form (sACE), and the other is expressed in germinal cells in the testes called testicular form (tACE) or germinal ACE (gACE) [7].
A polymorphism of the ACE gene by the insertion (I) or deletion (D) of a 287 bp Alu repeat sequence inside intron 16, which results in the three genotypes: Homozygotes I/I and D/D and heterozygotes I/D [8]. Although the polymorphism is located in a non-coding region, the D allele is associated with increased activity of ACE in serum [9]. There are highly inconsistent findings within all the major ethnic groups when the association of I/D polymorphism of ACE gene is studied with cardiovascular and renal complications and the risk for Type 2 Diabetes Mellitus (T2DM), where some studies have explained that the D allele is most frequent in T2DM and the associated complications in Tunisia [10] while others have found no association between ACE alleles frequencies with T2DM or cardiovascular disease (CVD) and renal disease in Malaysia and Indonesians [11]. The differences in the findings are largely due to the multifactorial or polygenic nature of these disorders, as evidenced by the variations of disease outcomes modulated by the between the gene to gene or gene to environment interactions [6].
There are more than 800 chronic kidney disease patients undergoing HD in Gaza Strip- Palestine, and approximately 40% of them have T2DM (personal conversation with Dr. Alqeshawi). To date, this is the first study on the association between ACE polymorphism with HD in Palestinian patients.
The objectives of the present study are: (a) To assess ACE genotypes frequency in the Palestinian population; (b) To evaluate ACE genotypes and allele frequency among HD patients and control subjects; (c) To investigate the relationship between ACE genotypes and HD risk in patients who have different clinical diseases.
Methods
Sampling
EDTA whole blood samples were collected from 186 individuals to conduct a retrospective case- control study. Eighty-six subjects (49 female and 37 male) undergoing HD for longer than six months at government hospitals from a total of 800 patients in Gaza Strip and 100 healthy individuals as control were included in this study (41 female and 59 male); participants were all Palestinian nationals above the age of 25 and were selected randomly. A self-administered questionnaire was completed by the participants (Appendix-A). DNA extraction from blood samples was performed by using a Promega kit and GeneAll kit for human DNA extraction by following the manufacturers’ instructions.
Ethical Considerations
Permission to conduct the study was obtained from the Ministry of Health in the Gaza Strip.
PCR Amplification of ACE Gene
Primers for ACE Gene Polymorphism
Polymerase chain reaction (PCR) was used to detect insertion or deletion of 287 bp Alu repetitive sequence closed to the 3’ end of intron 16 of the ACE gene for both groups (case and control). PCR oligonucleotide primers were used as described [9].
ACE gene Amplification and Temperature cycling program
Three µl (~150 ng) of the extracted DNA was added to 7 µl master mix (Bioline, UK), and 0.5 µl of each primer (5 pmol) in 0.2 ml thin-walled micro-centrifuge tubes. The tubes were centrifuged and then placed in a thermal cycler (Biometra, Germany).
PCR amplification was done according to the following thermal cycling conditions: Step 1. Denaturation for 1 minute at 95 C◦: Step 2. 36 cycles of melting for 15 seconds at 95 C◦; annealing for 15 seconds at 59 C◦; and extension for 10 seconds at 72 C◦; Step 3. Final extension for 10 minutes at 72◦C. PCR products were analyzed on 2% ethidium bromide-stained agarose gel electrophoresis to determine the polymorphism of the gene.
Data Analysis
The data were analyzed by using statistical package for Social Sciences (SPSS) version 18.0; Independent Samples t-test, Chi-square test and Odd’s Ratio (OR) were used to compare the case and control groups in this study. A p-value < 0.05 was considered to be statistically significant.
Results
Investigation of ACE gene I/D Polymorphism was conducted on a study group that included186 individuals who were separated into case and control groups: the case group consisted of 86 HD patients who had been undergoing dialysis for more than 4 months (mean age 56 ± 11.47) and the control group included 100 subjects (mean age 49.58± 7.87). The mean age for all subjects was (52.55 ± 10.20), where 79.04% of the study population were non-smokers, and 20.96% were smokers. Furthermore, 51.61% of the participants were males, and 48.38% were females. Eighty- six percent of HD patients were hypertensive, and 50.0% of them were diabetic, while the entire control population was non-hypertensive and non-diabetic.
PCR Results
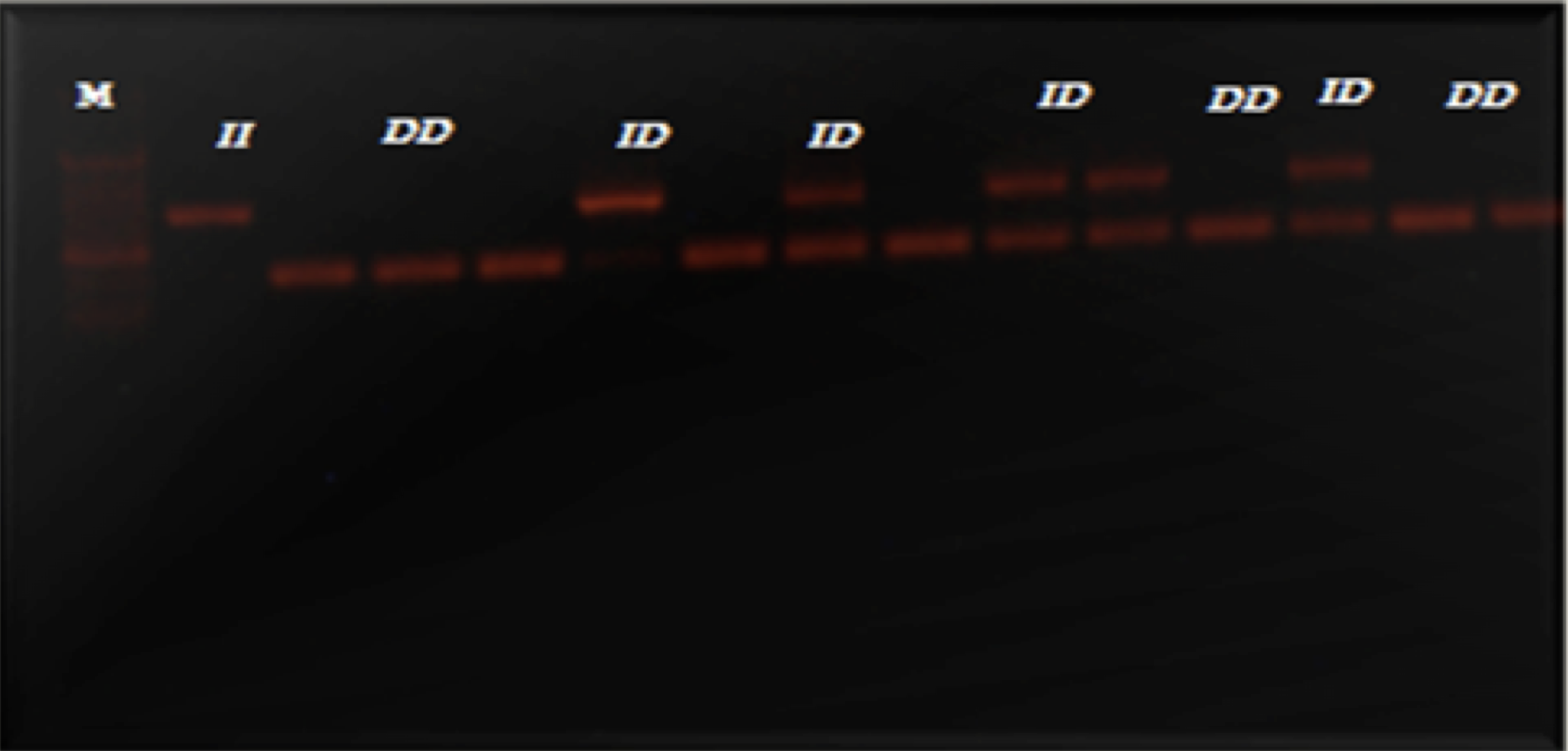
PCR against the ACE gene polymorphism was applied to detect ACE genotypes, 490 bp amplicon size was detected with I/I genotype and 190 bp amplicon to D/D genotype and the presence of two bands 490 bp and 190 bp showed the I/D genotype, as shown in Figure 1 . A negative control (with nuclease-free water instead of the DNA template) was included in each reaction. The size of the amplicon was estimated by comparing it with a DNA molecular size marker (50 bp ladder DNA) run on the same gel.
ACE Genotypes
ACE Genotypes frequency in the studied population
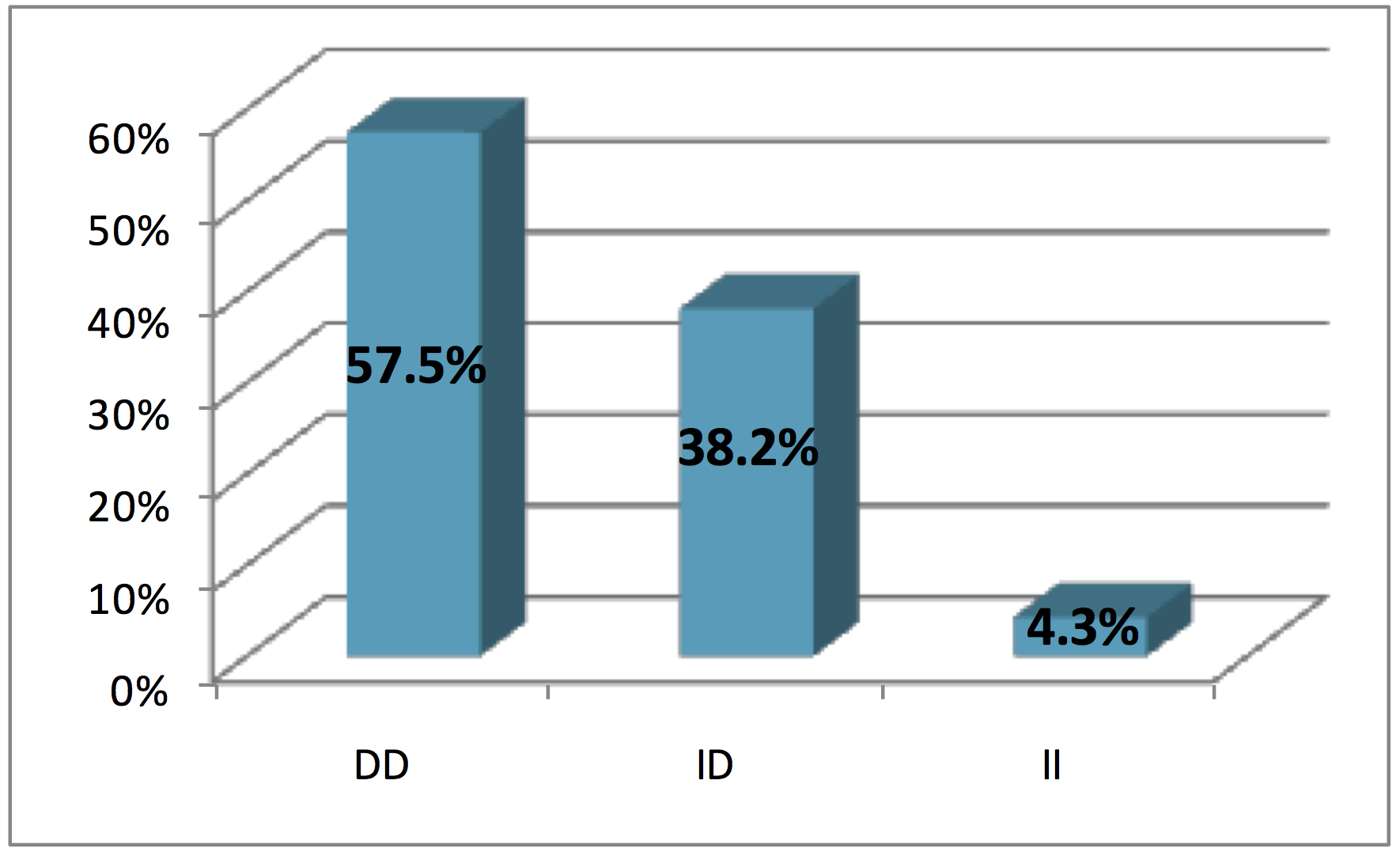
ACE genotypes distribution for all subjects were (57.5%) for D/D genotype, which was the most frequent among the studied population, followed by I/D genotype (38.2%), while I/I genotype was the lowest (4.3%), as shown in Figure 2 .
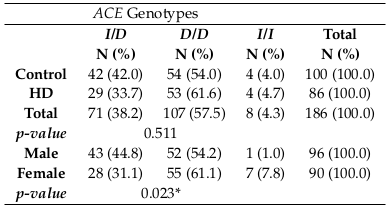
According to the p-value results, there were no statistically significant associations between the ACE genotypes and HD group or the control group (p-value = 0.511), but there was a significant difference between ACE genotypes and gender (p-value>0.05) Table 1 .
The frequency of the ACE allele in all subjects
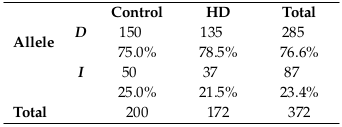
D allele was the most frequent allele among all subjects (76.6%), where it was found in 75.5% of the control group and 78.5% of the HD patients, while I allele was (23.4%), with 25.0% in control and 21.5% in HD patients, as shown in Table 2 .
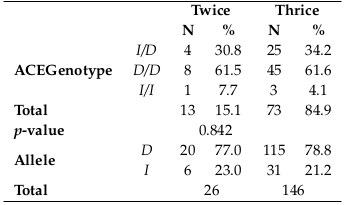
Relationship between ACE genotypes in HD patients with dialysis frequency per week.
The data showed that there was no statistically significant relationship between ACE genotypes and the weekly dialysis frequency in HD patients (p-value > 0.05) Table 3 .
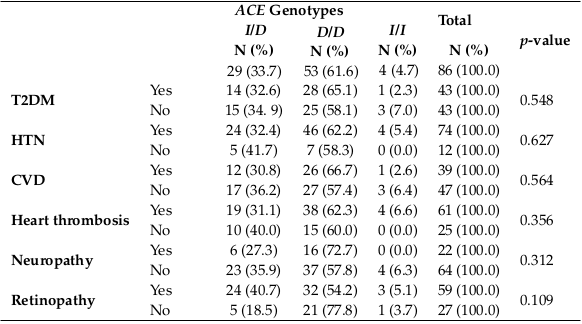
Relationship between ACE genotypes with T2DM, HTN, neuropathy, CVD, retinopathy, and thrombus in HD patients.
When the relationship between ACE genotypes and T2DM, HTN, neuropathy, CVD, and retinopathy in HD patients were tested, no statistically significant relationship was clarified, as shown in Table 4 . D/D genotype was the most frequent genotype in each of the clinical diseases separately, and this may be due to the increase of D/D genotype frequency in all study subjects.
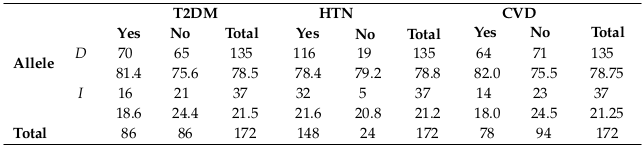
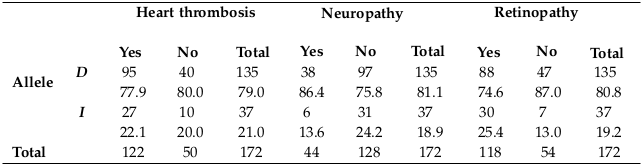
Distribution of ACE allele frequency in HD patients with T2DM, HTN, CVD, heart thrombosis, neuropathy, and retinopathy
D allele was the highest frequency in all subjects and also in each clinical condition separately, as shown in Table 5 and Table 6 , and this may be related to the frequency percentage in each genotype.
As the table above shows, D allele was the most frequent allele in T2DM, HTN and CVD patients who were undergoing dialysis. Also, as shown in the previous table, D allele was the most frequent allele in heart thrombosis, neuropathy and retinopathy patients who were undergoing dialysis.
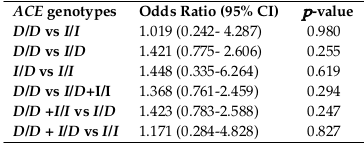
(vi) Odds Ratio (OR) and 95% Confidence Interval (CI) of ACE genotypes between HD patients and control group.
A comparison was made to test the relationship between ACE genotypes and HD. The OR, CI and -value were calculated for each associate, as shown in Table 7 . The OR of the D/D genotype in the HD group was (1.421) times the control population when compared to the I/D genotype, although this was not statistically significant and was the same as the other genotypes; there was an increase in the risk, but this increase was not sufficient to make the relationship statistically significant.
Discussion
Chronic kidney disease is distinguished by renal destruction with or without renal function loss, correlated to the high morbidity and mortality over the continuum from early to advanced stages, which requires renal replacement therapy. Although the exact cause of CKD is not always known, any condition or disease that damages blood vessels or other structures in the kidneys can lead to kidney disease. Diabetes and HTN are the most common causes of CKD that leads to kidney failure, and they may also accelerate the progression of chronic kidney disease in someone who already has the disease.Despite the numerous techniques that are used to prevent, diagnose and also treat CKD, it is considered to be a public health disease that requires attention and monitoring.
The RAAS is considered as one of the most important regulatory systems for cardiovascular homeostasis. Ang II, which is cleaved from Ang I by ACE, is a vasoconstrictor and growth stimulator when acting on the Ang II type 1 receptor (AT1). Consequently, ACE inhibitors and AT1 blockers decrease blood pressure and have beneficial effects in cardiovascular and renal disease, and the effects of ACE inhibitors on renal hemodynamics vary widely depending on the pre-existing physiologic and pathologic state of the kidneys.
The results showed that ACE Genotypes frequencies were 57.5% for D/D, 38.2% for I/D and 4.3% for I/I genotype. ACE genotype distribution differs from one population to another or even from one year to another in the same population. In Palestine, the D/D genotype was the most frequent genotype as demonstrated by the present study and this result is also similar to other studies [8][12], as it was shown to be the most frequent genotype in Iraq [13], while the I/D genotype was the most frequent genotype in Turkey [14] and the UAE [6]. Some countries like Egypt [15,16] have an inconstant frequency for the genotypes, and this may be related to the sample size, the population of the study or the techniques used in the determination of ACE gene polymorphism. However, other countries the distribution of the genotypes is constant, such as Palestine [12] and Iraq [13], also in this study ACE alleles frequency compared to different studies, where D allele was more frequent in this study and other studies [6][12]-[14], whereas the I allele had the highest frequency in another study [16].
Additionally, the results revealed that the association between ACE genotypes with HD was not statistically significant and this result is similar to a study conducted in China [17]. Although the I/D genotypes where the most frequent in [17] and D/D was the most frequent in this study, both studies did not find a significant association between ACE genotypes and HD. On the other hand, in a meta-analysis study found that the development of nephropathy is not associated with the presence of D allele in the Asian and Caucasian populations; only the D/D genotype could be associated with the development of nephropathy (1.96 of risk) [18], while the results of this study revealed an increase in nephropathy development risk for D/D genotypes but the relationship is not statistically significant OR (1.368) and CI (0.761-2.459) for D/D genotype vs. I/D and I/I.
The two main causes of CKD are diabetes and high blood pressure, which are responsible for up to two-thirds of the cases. One of the most frequent complications of diabetes is nephropathy which progressively leads to HD. Type 2 diabetic nephropathy (T2DN) is defined as persistent albuminuria in type 2 diabetic individuals for more than five years with many changes in renal function such as an increase in renal blood flow, glomerular hyperfiltration, kidney hypertrophy and increased albumin excretion rate [19].
In this study, there was no statistically significant association between ACE genotypes with HD in T2DM patients and this is similar to other studies such as in Tunisia [20], but is dissimilar to other studies conducted in Tunisia [10], Egypt [16] and Iraqi [13], which found an increase inT2DM risk in D/D genotype individuals.
In terms of the association with HTN, it was not deemed to be statistically significant, and this result concurs with [8] and [14] but disagrees with Alsafar et al. (2015) [6]. D/D genotype was the most frequent genotype in both diabetes and HTN patients, and this genotype is associated with a 2-fold increase in ACE activity, whereas those with the I/I genotype have the lowest ACE expression. The physiological importance of this polymorphism is its association with plasma ACE activity and the increase of ACE levels in the blood which is related to the D/D genotype and disturbs normal kidney function, as reported in different studies [9][21][22]. Thus, the evaluation of ACE I/D polymorphism may be a beneficial tool to identify patients at risk and those who may benefit the most from Reno-protective therapy with ACE inhibitors. Furthermore, it may guide pharmacologic therapy in individual patients and help design clinical trials in progressive nephropathies. Moreover, it could be helpful for optimizing prevention and intervention strategies at population levels, as reported in Ruggenenti et al. (2008) [23], also its reported that characterization of the ACEI/D gene polymorphism has been suggested for decision making in relation to antihypertensive treatment regimens [24].
In regard to the association of ACE genotypes with CVD or heart thrombosis, it was not statistically significant, and this is in concordance with Saqer et al. (2016) [12] , but not with a study conducted in Saudi Arabia study [25], which found an increased CVD risk with ACE I/D polymorphism. The relationship between ACE genotypes and retinopathy was also not statistically significant, and this is similar to another study conducted in Iran [26]. In terms of the relationship between ACE genotypes and neuropathy, there was no statistically significant relationship, which contradicts a meta-analysis study [27] that clarified the relationship between neuropathy risk and ACE gene I/D polymorphism. The multifactorial or polygenic nature of these diseases may explain the inconstancies between this study and previous studies.
Conclusion
The findings showed that D/D genotype (57.5%) was the most common in all subjects in the Gaza Strip. Additionally, the frequency of D allele was (76.6%) in all subjects, and the frequency of I allele was (23.4%) in all subjects. These findings suggest that there is no direct correlation between ACE genotypes and HD and T2DM, HTN, CVD, heart thrombosis, neuropathy, while a partial association could perhaps be explained by the nature of multifactorial or polygenic diseases. Increased dosage of ACE inhibitors in patients with D/D genotype for ACE gene could be a more effective treatment option.
The original PCR method [9] has been reported to sometimes miss the extension of I allele, particularly in heterozygotes. Improved methods, which include a second, nested extension with an I allele-specific primer, have been designed for this reason. In a future study, the genotypes should be checked with such a method.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
CKD: Chronic Kidney Disease; GFR: Glomerular Filtration Rate; ACE: Angiotensin Converting Enzyme; HD: Hemodialysis; ERD: End-Stage Renal Disease; CVD: Cardiovascular disease; HTN: hypertension; RAAS: Renin Angiotensin Aldosterone System; ACE: Angiotensin Converting Enzyme; AngI: Angiotensin I; AngII: Angiotensin II; sACE: somatic ACE; gACE: germinal ACE; I/D: Insertion/Deletion
Ethics approval and consent to participate
Permission to conduct the study was obtained from the Ministry of Health in the Gaza Strip. The permission name: Facilitate the task of the students to do graduate research / Asmaa Abuaisha, 04/05/2016.
Availability of data and materials
A self-administered questionnaire for the participants is available at here (Appendix-A)
Competing interests
The authors declare that no competing interests exist.
Authors’ contributions
All Authors participated in drafting the article and revising it critically for important intellectual content, also they all gave a final approval of the version to be submitted. Asmaa Mahmoud Abuaisha, Lamia Faisal Abou Marzoq, Mai Sufian Eljbour, Eman Saad Fayyad and Abeer Kamal Baraka are clinical and experimental investigators. Nedime Serakinci served as scientific advisors.
References
-
L
Carroll.
The Stages of Chronic Kidney Disease and the Estimated Glomerular Filtration Rate. 2006;
1
:
64-9
.
-
PW
Mathieson.
The cellular basis of albuminuria. Clinical Science.
2004;
(London
:
England) 107
.
View Article PubMed Google Scholar -
M
Rhodes.
Hemodialysis, Web. MD Medical Reference from. Health. wise.
2009
.
-
T
Ishimitsu,
K
Tsukada,
S
Ohta,
H
Inada,
J
Minami,
H
Ono.
Increased cardiovascular risk in long-term hemodialysis patients carrying deletion allele of ACE gene polymorphism. American Journal of Kidney Diseases.
2004;
44
:
466-75
.
View Article Google Scholar -
R
Pontremoli,
M
Ravera,
F
Viazzi,
C
Nicolella,
V
Berruti,
G
Leoncini.
Genetic polymorphism of the renin-angiotensin system and organ damage in essential hypertension. Kidney International.
2000;
57
:
561-9
.
View Article PubMed Google Scholar -
H
Alsafar,
A
Hassoun,
S
Almazrouei,
W
Kamal,
M
Almaini,
U
Odama,
N
Rais.
Association of angiotensin converting enzyme insertion-deletion polymorphism with hypertension in emiratis with type 2 diabetes mellitus and its interaction with obesity status. Disease Markers 2015.
2015
.
-
H
El-Dorry,
C
Pickett,
J
MacGregor,
R
Soffer.
Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proceedings of the National Academy of Sciences of the United States of America.
1982
.
-
L
Saqer.
ACE I/D polymorphism in hypertensive patients of Palestinian population. 2016;
4
:
6-10
.
-
B
Rigat,
C
Hubert,
F
Alhenc-Gelas,
F
Cambien,
P
Corvol,
F
Soubrier.
An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. The Journal of Clinical Investigation.
1990;
86
:
1343-6
.
View Article PubMed PMC Google Scholar -
T
Baroudi,
R
Bouhaha,
C
Moran-Moguel,
J
Sanchez-Corona,
HB
Maiz,
HK
Abid.
Association of the insertion/deletion polymorphism of the angiotensin-converting enzyme gene with type 2 diabetes in two ethnic groups of Jerba Island in Tunisia. Journal of the Renin-Angiotensin-Aldosterone System.
2009;
10
:
35-40
.
View Article PubMed Google Scholar -
H
Sinorita,
M
Madiyan,
R
Pramono,
L
Purnama,
M
Ikhsan,
A
Asdie.
ACE gene insertion/deletion polymorphism among patients with type 2 diabetes, and its relationship with metabolic syndrome at Sardjito Hospital, Yogyakarta, Indonesia. Acta Medica Indonesiana.
2010;
1
:
12-6
.
-
L
Saqer,
H
Khammash,
E
Shurrab,
M
Aabed,
R
El-Malakh.
Association between angiotensin converting enzyme gene insertion deletion polymorphism and coronary heart disease in Gaza Strip. 2016;
4
:
18-26
.
-
S
Al-Awadi,
A
Ghareeb,
A
Oleiwi,
W
Salo,
A
Moner.
Genotype Distribution of Angiotensin I- Converting Enzyme in Iraqi Arab Population. 2011;
4
:
24-9
.
-
S
Inanir,
S
Yigit,
F
Camcelike,
O
Ates,
SE
Taycan,
Y
Nursal.
Relationship between major depressive disorder and ACE gene I/D polymorphism in a Turkish population. Archives of Clinical Psychiatry.
2016;
43
:
27-30
.
View Article Google Scholar -
L
Rashed,
RA
Hay,
R
Mahmoud,
N
Hasan,
A
Zahra,
S
Fayez.
Association of angiotensin converting enzyme gene polymorphism with inflammation and cellular cytotoxicity in vitiligo patients. PLoS One.
2015;
10
:
e0132915
.
View Article PubMed PMC Google Scholar -
O
Shaker,
M
Ismail,
E
Ashour,
H
Yousif,
M
Afify,
W
Gouda.
ACE gene polymorphism and serum ACE level with Progression of Nephropathy in Type 2 Diabetic Patients. 2014;
9
:
2023-32
.
-
AY
Wang,
JC
Chan,
M
Wang,
E
Poon,
SF
Lui,
PK
Li.
Cardiac hypertrophy and remodeling in relation to ACE and angiotensinogen genes genotypes in Chinese dialysis patients. Kidney International.
2003;
63
:
1899-907
.
View Article PubMed Google Scholar -
FP
Schena,
C
D'Altri,
G
Cerullo,
C
Manno,
L
Gesualdo.
ACE gene polymorphism and IgA nephropathy: an ethnically homogeneous study and a meta-analysis. Kidney International.
2001;
60
:
732-40
.
View Article PubMed Google Scholar -
S
T,
G
V..
Diabetic nephropathy. Medicine.
2006;
34
:
83-86
.
View Article Google Scholar -
I
Arfa,
A
Abid,
S
Nouira,
H
Elloumi-Zghal,
D
Malouche,
I
Mannai.
Lack of association between the angiotensin-converting enzyme gene (I/D) polymorphism and diabetic nephropathy in Tunisian type 2 diabetic patients. Journal of the Renin-Angiotensin- Aldosterone System.
2008;
9
:
32-6
.
View Article PubMed Google Scholar -
S
Eleni,
K
Dimitrios,
P
Vaya,
M
Areti,
V
Norma,
G
Magdalini.
Angiotensin-I converting enzyme gene and I/D polymorphism distribution in the Greek population and a comparison with other European populations. Journal of Genetics.
2008;
87
:
91-3
.
View Article Google Scholar -
JB
Zhou,
JK
Yang,
JK
Lu,
YH
An.
Angiotensin-converting enzyme gene polymorphism is associated with type 2 diabetes: a meta-analysis. Molecular Biology Reports.
2010;
37
:
67-73
.
View Article PubMed Google Scholar -
P
Ruggenenti,
P
Bettinaglio,
F
Pinares,
G
Remuzzi.
Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clinical Journal of the American Society of Nephrology; CJASN.
2008;
3
:
1511-25
.
View Article PubMed PMC Google Scholar -
WY
So,
RC
Ma,
R
Ozaki,
PC
Tong,
MC
Ng,
CS
Ho.
Angiotensin-converting enzyme (ACE) inhibition in type 2, diabetic patients - interaction with ACE insertion/deletion polymorphism. Kidney International.
2006;
69
:
1438-43
.
View Article PubMed Google Scholar -
KM
Al-Harbi,
IS
Almuzaini,
MM
Morsy,
NA
Abdelaziz,
AM
Al-Balawi,
AM
Abdallah.
Angiotensin-converting enzyme gene insertion/deletion polymorphism in Saudi patients with rheumatic heart disease. Saudi Medical Journal.
2015;
36
:
176-80
.
View Article PubMed PMC Google Scholar -
A
Nikzamir,
A
Rashidi,
A
Esteghamati,
M
Nakhjavani,
T
Golmohammadi,
O
Khalilzadeh.
The relationship between ACE gene insertion/deletion polymorphism and diabetic retinopathy in Iranian patients with type 2 diabetes. Ophthalmic Genetics.
2010;
31
:
108-13
.
View Article PubMed Google Scholar -
W
Xu,
Y
Qian,
L
Zhao.
Angiotensin-converting enzyme I/D polymorphism is a genetic biomarker of diabetic peripheral neuropathy: evidence from a meta-analysis. International journal of clinical and experimental medicine.
2015;
8
:
944
.
PubMed PMC Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 4 (2018)
Page No.: 2160-2170
Published on: 2018-04-18
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3754 times
- Download PDF downloaded - 1273 times
- Appendix A downloaded - 0 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress