Abstract
Background: Although using Doppler ultrasonography of umbilical structures is a common method for predicting prenatal adverse outcome, recent studies have proposed the importance of middle cerebral artery (MCA) Doppler assessment for antenatal monitoring. The aim of the present study was to compare alterations in the fetalMCAversus umbilical artery (UA) pulsatility indices (PI) in complicated pregnancies by fetal growth restriction (FGR).
Methods: A cohort study was carried out in Yas Hospital (Tehran, Iran) in 2016. One hundred fifty pregnant women in third trimester with estimation of FGR participated in the study. Transabdominal ultrasound was performed to determine fetal weight. After birth, all neonates were divided into 2 groups: small for gestational age (SGA) and non-SGA.We compared the values of UA and MCA Doppler in predicting SGA. Data were analyzed with t-test and Chi-square test.
Results: After delivery, 126 mothers had SGA while 24 subjects had non-SGA neonates. Unlike UA PI, MCA artery PI of the SGA and non-SGA groups were significantly different (p=0.062 for UA PI comparison; p=0.0001 for MCA PI comparison). Of the 126 cases, there was decreased MCA PI in 40.5% of fetuses and increased UA PI in 22.2% of fetuses. There were significant differences in both sensitivity and specificity for MCA PI versus UA PI (p=0.014 for sensitivity; p=0.009 for specificity).
Conclusion: Results showed that of all the SGA cases, a decrease in MCA PI was more notable than changes in UA PI. Sensitivity, specificity and prediction of SGA for MCA PI were higher than those for UA PI.
Background
Fetal growth restriction (FGR) is defined as failure to achieve specific fetal biometric measures or estimated weight (less than 10th percentile) by a specific gestational age. FGR is responsible for different types of pre- and post-natal morbidity and mortality. The prevalence of FGR is reported to be about 3-10%. Based on the time of onset, it is classified into 2 groups- early (before 32 weeks) and late (after 32 weeks) onset. Some factors leading to FGR include maternal causes (hypertension, diabetes, cardiopulmonary disease, anemia, malnutrition, smoking, drug use), fetal causes (genetic disease including aneuploidy, congenital malformations, fetal infection, multiple pregnancies), and placental causes (placental insufficiency, placental infarction, placental mosaicism) [1]–[5].
In the past, evaluation of an FGR fetus was performed by utilizing electronic fetal monitoring and biophysical profile. These methods have several disadvantages, including false positive findings which lead to waste of time [6]. Nowadays, Doppler velocimetry examination is an improved clinical tool for assessing utero-placental blood flow and resistances in complicated pregnancies by preeclampsia or FGR [7].
Some studies have demonstrated that diagnosis and management of FGR rely on umbilical artery Doppler (UAD) and others have shown the benefits of uterine artery Doppler assessment in the first-trimester development of FGR. The value of combining pulsatility indices (PI) of both the umbilical artery (UA) and uterine artery for improving prediction of FGR has also been demonstrated. On the other hand, several studies have indicated that there is no correlations between fetal Doppler indices and predictive value for small for gestational age (SGA) [8]–[12].
The middle cerebral artery (MCA) may also predict fetal outcomes from alterations in cerebral blood flow and its direction [13]. It was confirmed that during the prenatal period, the resistance in the cerebral artery of the fetus is high. However, this parameter can change in threatening conditions, such as placental insufficiency and hypoxemia, due to stimulation of chemoreceptors and alteration in vasodilator or vasoconstrictor agents [14]. MCA Doppler measurement has been advocated as an efficient modality for detection of fetal hypoxia, which leads to perinatal adverse outcome and fetal compromise [15].
Prediction of SGA is valuable for earlier prevention and chance for better treatment outcome. 3 The purpose of the present study was to compare the values of UA versus MCA Doppler in predicting late onset FGR to potentially modify obstetrical management.
Methods
Study population and study design
This prospective cohort study was carried out at Yas Hospital, affiliated with Tehran University of Medical Sciences (Tehran, Iran) in 2016. The target population included pregnant women in their third trimester, with estimation of fetal FGR by ultrasound examination. The inclusion criteria included fetal growth < the 10th percentile for weight of all fetuses at the same gestational age, gestational age 32+0 to 39+6 weeks, singleton pregnancy, and no major fetal anomalies. Exclusion criteria included premature rupture of membrane, post term pregnancy, mother’s decline, and development of pre-eclampsia. All participants gave written consent and were accepted; they received routine prenatal care and delivery in our center.
Transabdominal ultrasound was performed to determine fetal weight. If the estimated weight was under the 10th percentile according to the Hadlock formula [16], Doppler ultrasound of the UA and MCA was done. Ultrasound examination was carried out by an expert perinatologist (who was blinded to the study) using Doppler and high resolution ultrasonography (ACUSON Sequoia 512TM, Siemens Healthcare GmbH, made in USA). UA PI in the upper 95th percentile was considered as abnormal range (values more than 1.2). MCA PI was measured 2 mm upper the internal carotid, in the axial plan, and the angle between the Doppler beam and the vessel was set at 0-30. MCA PI under the 5th percentile was considered as abnormal range (values less than 0.9).
Immediately after birth, for determination of arterial blood gas, 1-2 ml of neonatal blood was drawn from UA with a heparinized syringe, labeled, and transferred to the laboratory. All neonates were divided into the 2 groups: SGA and non-SGA, based on the Hadlock formula. Data related to the MCA and UA PI indices were compared between the 2 groups. Finally, we compared the values of UA and MCA Doppler in predicting SGA. Moreover, correlations between related factors between the 2 groups were evaluated.
Participant characteristics
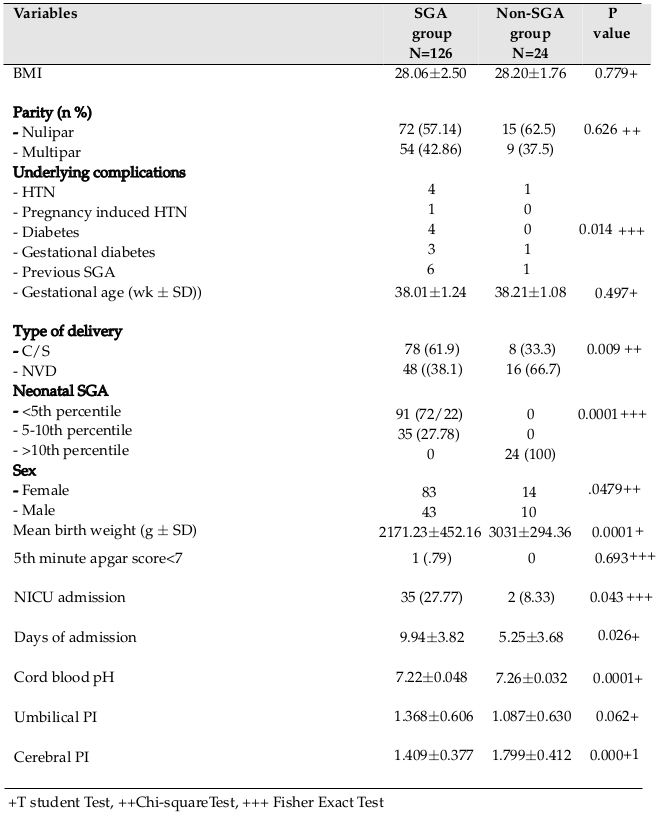
Demographic characteristics of the mothers and their neonates included maternal age, body mass index (BMI), gestational age, parity, type of delivery, underlying diseases, birth weight of the neonates, sex, 5-minute Apgar score, arterial blood gas (ABG) results, neonatal complications, cause of hospitalization, days of neonatal intensive-care unit (NICU) admission, UA PI, and MCA PI. All information were gathered and recorded on checklists.
Data analysis
The software package SPSS version 20 was used to perform the statistical analysis. Data are presented as mean + standard deviation for continuous variables and frequency/percentage for categorical variables. The t-test and Chi square test were applied to analyze the relationships between variables. P value < 0.05 was considered as statistical significance.
Ethical considerations
All gathered data were considered confidential. No extra cost was constrained on participants. All participants signed an informed consent. Moreover, subjects were assured about their rights to discontinue the study course. Ethics approval for the study was obtained from the Institutional Review Board of Tehran University of Medical Sciences, according to the Helsinki declaration 4 (No; IR. TUMS MEDICINE REC, 1395; 1264).
Results
One hundred fifty pregnant women with estimated fetal FGR (based on ultrasound findings) participated in the study. After delivery, 126 mothers (84%) had an SGA neonate while 24 subjects (16%) had a non-SGA neonate. The mean maternal age in the SGA versus Non-SGA groups were 27.83±5.85 and 27.45±5.55 years, respectively. Independent t-test analysis did not show any significant differences between both groups regarding maternal age (p=0.51). No neonatal mortality was seen and no neonates needed ventilation.
Our results showed that cesarean section in the SGA group was significantly more frequent than in the non-SGA group (p=0.00). There was a significant correlation between maternal underlying disease and delivery of a SGA neonate (p=0.01). Mean birth weight in the SGA group was significantly lower than in the non-SGA group, and most neonates in this group had a neonatal weight below the 5th percentile for gestational age (p=0.00). More neonates in the SGA group were hospitalized in the NICU and stayed longer compared to neonates in the other group (p=0.04). There was a significant difference between the 2 groups with regard to mean cord blood pH (in the SGA group, 7.22±0.048, in the non-SGA group; 7.26±0.032, p=0.00). Detailed data of subjects and their neonates are shown in Table 1 .
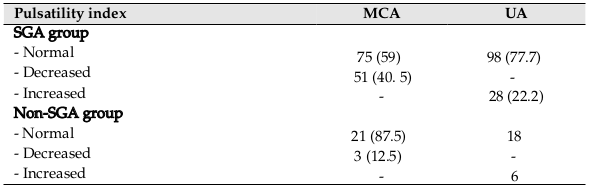
With regard to Doppler examination, MCA PI between SGA and non-SGA groups was significantly different. However, there was no significant difference between 2 groups as it pertains to UA PI (p=0.00 vs. p=0.06) ( Table 1 ). Table 2 shows detailed data related to the MCA and UA indices. Of the 126 cases, decreased MCA PI as observed in 40.5% of fetuses, and increased UA PI was observed in 22.2% of fetuses. Of all, 47 cases showed abnormal PI in one of 2 arteries and 16 cases showed abnormality in both arteries. Cord blood pH in SGA neonates with abnormal PI (MCA or UA) was significantly lower than that in SGA neonates with normal PI (7.20±0.012 vs. 7.24±0.051, p=0.01). On the other hand, in the non-SGA group, this association was not significant (blood pH in neonates with abnormal PI=7.25±0.044 blood pH in neonates with normal PI=7.26±0.028, p=0.26).
Moreover, the study results showed significant correlations between NICU hospitalization and abnormality for both MCA and UA PI. Indeed, of 150 neonates, 96 cases had normal MCA PI, of which 9 needed NICU hospitalization. Meanwhile, 54 neonates had abnormal MCA PI, and 28 cases were hospitalized (p=0.00). Furthermore, of the 116 neonates with normal UA PI, 21 cases were hospitalized while 34 had abnormal UA PI, of which 16 cases needed NICU hospitalization (p=0.00).
Detailed analysis also demonstrated that of the 126 SGA neonates, NICU hospitalization was more frequent in neonates with abnormal MCA PI than in those with normal MCA PI (54.9% vs. 9.3%, p=0.0001). This positive correlation was also significant for abnormal UA PI versus normal UA PI (57.1% vs. 19.4%, p=0.00). On the other hand, the associations between the same variables were not notable among neonates in the non-SGA group.
We also found that the frequency of neonatal respiratory distress syndrome (RDS) among SGA neonates with abnormal MCA PI was significantly higher than in neonates with normal MCA PI (p=0.01). On the other hand, the frequency of RDS was not different among non-SGA neonates when comparing normal versus abnormal MCA PI. Moreover, the frequency of RDS between SGA (3.2%) and non-SGA groups (4.2%) was not different.
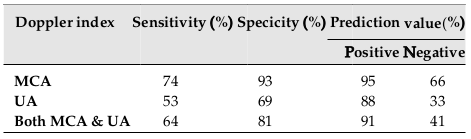
There was a significant correlation between cerebroplacental ratio (CPR) and MCA PI; however, this correlation was not observed for CPR and UA PI (p=0.00 vs. p=0.95, respectively). The results showed CPR <1 as a potential predictor of adverse outcome among SGA neonates ( Table 3 ). Table 4 shows the sensitivity, specificity, and prediction value for both MCA PI and UA PI. There were significant differences for sensitivity and specificity between MCA PI and UA PI (p=0.01; p=0.00, respectively. The study results revealed a significant lower value for the UA PI index as compared to the MCA PI for SGA prediction.
Discussion
Although using Doppler ultrasonography of UA is a common method for evaluation of uteroplacental circulation and predicting perinatal adverse outcomes [8]–[11], recent studies have proposed the importance of MCA Doppler assessment for antenatal monitoring [17]. The present study compared the alterations in fetal MCA and UA PI in complicated pregnancies (by FGR). To the best of our knowledge, this study is among the first few studies focused on SGA predicting values for MCA and UA PI.
The most important finding of the present study was that of the 126 SGA cases, a decrease in MCA PI was statistically more notable than changes in UA PI (40.5% vs. 22.2%, respectively). Consistent with our findings, other investigation also revealed that diastolic blood flow (indicating redistribution of the blood to vital organs) appears earlier in fetal cerebral circulation than in the UA artery and aorta. Moreover, alterations in cerebral circulation are more sensitive to changes in the systemic circulation, that can predict the condition of the fetus [17].
In accordance with other investigations, the obtained results showed substantial risks for adverse perinatal outcomes due to FGR. The frequencies of cesarean delivery, low birth weight, longer hospitalization and acidosis were more common among SGA neonates, as opposed to non-SGA neonates. Higher frequencies of stillbirth, neonatal death, and complications related to prematurity (such as neurocognitive delay, metabolic syndrome, and cardiovascular disease) were seen among neonates with FGR [18]. Nanthakomon et al. also revealed that SGA neonates with abnormal UA PI had high frequency of NICU admission and long hospitalization. Moreover, among the SGA neonates with abnormal MCA PI, low birth weight, hospitalization at NICU, need for ventilators, and 5-min Apgar score <7, were all common [19].
However; we could not find any correlation between SGA and 5-min Apgar score <7. Consistent to our finding, Nannig et al. have also demonstrated low levels of pH in 43 SGA neonates [20]. Sharma et al. revealed the importance of maternal conditions like maternal health, behavioral habits, maternal underlying disease, and mismatch between the supply of nutrients by the placenta and the demand of the fetus in FGR etiology [21]. Werner et al. showed that of 2885 SGA neonates, delivery by cesarean was more common than by normal vaginal delivery (57.9% vs. 42.1%, p<0.05) [22].
Such common adverse perinatal outcomes demonstrate the importance of earlier diagnosis of SGA and interventions. According to our results, a significant correlation was observed between CPR and MCA PI. CPR <1 were found as a potential predictor of adverse outcome among SGA neonates. The results also showed a high sensitivity, specificity and predicting value of middle cerebral PI (74%, 93% and 95%, respectively) in SGA prediction. Our finding was compatible with other studies; Hemlata et al. demonstrated that 71%, 92% and 94% represented the sensitivity, specificity, and positive predictive value of MCA for detecting SGA-related abnormal fetal outcomes [23]. Ibrahim et al. also revealed a lower PI value of MCA as a highly specific value for predicting FGR and fetal demise [24]. Monteith et al. have shown the importance of a serial abnormal CPR <1 as a potential predictor of adverse outcomes among 1116 SGA fetuses [25].
Finally, the results of the present study showed false positive errors related to ultrasound examination in predicting fetal weight. Although 150 fetuses were diagnosed as FGR in the third trimester, after delivery 24, neonates were not SGA. In accordance with our results, Williams et al. demonstrated that there was about 20% false positives and 20% false negatives in sonographic estimation of fetal weight [26].
Our study included a few limitations. First, the data were limited to the third trimester of gestation. Second, we did not consider other maternal variables, such as ethnicity and smoking, which may influence the findings. Indices related to uterine artery were not considered, though they could provide informative data. Finally, for evaluation of different factors, a larger sample size is needed.
Conclusion
Our study results showed that of the SGA cases, a decrease in MCA PI was statistically more notable than changes in UA PI. Sensitivity, specificity and predictive values of MCA PI for SGA prediction was higher than those for UA PI.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
FGR: Fetal growth restriction; MCA: middle cerebral artery; PI: pulsatility indices ; UA: umbilical arteries ; UAD: umbilical artery Doppler
Ethics approval and consent to participate
Ethics approval for the study was obtained from the Institutional Review Board of Tehran University of Medical Sciences, according to the Helsinki declaration (No; IR. TUMS MEDICINE REC, 1395; 1264).
Availability of data and materials
Will be provided if requested.
Competing interests
The authors declare that there is no conflict of interests.
Funding
None.
Authors’ contributions
Dr. Rahimi and Teimoory carried out the design and coordinated the study, participated in most of the experiments. Dr. Pirjani, Movahed and Shariat coordinated and carried out all the experiments, Analysis of data and participated in manuscript preparation. Mrs. Farahani provided assistance for all experiments and prepared the manuscript. All authors have read and approved the content of the manuscript.
References
-
J
Henrichs,
V
Verfaille,
L
Viester,
M
Westerneng,
B
Molewijk,
A
Franx,
HVD
Horst,
JE
Bosmans,
AD
Jonge,
P
Jellema.
Effectiveness and cost-effectiveness of routine third trimester ultrasound screening for intrauterine growth restriction: study protocol of a nationwide stepped wedge cluster-randomized trial in The Netherlands (The IRIS Study). BMC pregnancy and childbirth.
2016;
16
:
310
.
View Article Google Scholar -
D
Muresan,
IC
Rotar,
F
Stamatian.
The usefulness of fetal Doppler evaluation in early versus late onset intrauterine growth restriction. Review of the literature. Medical ultrasonography.
2016;
18
:
103
.
View Article PubMed Google Scholar -
Z
Fardiazar,
S
Atashkhouei,
Y
Yosefzad,
M
Goldust,
R
Torab.
Comparison of fetal middle cerebral arteries, umbilical and uterin artery color Doppler ultrasound with blood gas analysis in pregnancy complicated by IUGR. Iranian journal of reproductive medicine.
2013;
11
:
47
.
PubMed PMC Google Scholar -
H
Rasyid,
S
Bakri.
Intra-uterine Growth Retardation and Development of Hypertension. Acta Medica Indonesiana.
2016;
48
:
320-324
.
PubMed Google Scholar -
A
Suhag,
V
Berghella.
Intrauterine growth restriction (IUGR): etiology and diagnosis. Current Obstetrics and Gynecology Reports.
2013;
2
:
102-111
.
View Article Google Scholar -
AA
Baschat,
HL
Galan,
A
Bhide,
C
Berg,
ML
Kush,
D
Oepkes,
B
Thilaganathan,
U
Gembruch,
CR
Harman.
Doppler and biophysical assessment in growth restricted fetuses: distribution of test results. Ultrasound in obstetrics & gynecology.
2006;
27
:
41-47
.
View Article PubMed Google Scholar -
J
Piazze,
S
Gioia,
A
Cerekja,
G
Larciprete,
T
Argento,
S
Pizzulo,
EV
Cosmi.
Doppler velocimetry alterations related to platelet changes in third trimester pregnancies. Platelets.
2007;
18
:
11-15
.
View Article PubMed Google Scholar -
K
Levytska,
M
Higgins,
S
Keating,
N
Melamed,
M
Walker,
NJ
Sebire.
Placental pathology in relation to uterine artery Doppler findings in pregnancies with severe intrauterine growth restriction and abnormal umbilical artery Doppler changes. American journal of perinatology.
2017;
34
:
451-457
.
PubMed Google Scholar -
K
Melchiorre,
K
Leslie,
F
Prefumo,
A
Bhide,
B
Thilaganathan.
First-trimester uterine artery Doppler indices in the prediction of small-for-gestational age pregnancy and intrauterine growth restriction. Ultrasound in Obstetrics & Gynecology.
2009;
33
:
524-529
.
View Article PubMed Google Scholar -
S
Gudmundsson,
K
Flo,
G
Ghosh,
T
Wilsgaard,
G
Acharya.
Placental pulsatility index: a new, more sensitive parameter for predicting adverse outcome in pregnancies suspected of fetal growth restriction. Acta obstetricia et gynecologica Scandinavica.
2017;
96
:
216-222
.
View Article PubMed Google Scholar -
T
Nagar,
D
Sharma,
M
Choudhary,
S
Khoiwal,
RP
Nagar,
A
Pandita.
The role of uterine and umbilical arterial doppler in high-risk pregnancy: a prospective observational study from India. Clinical Medicine Insights: Reproductive Health.
2015;
9
:
CMRH-S24048
.
-
S
Triunfo,
M
Parra-Saavedra,
V
Rodriguez-Sureda,
F
Crovetto,
C
Dominguez,
E
Gratacós,
F
Figueras.
Angiogenic factors and Doppler evaluation in normally growing fetuses at routine third-trimester scan: prediction of subsequent low birth weight. Fetal diagnosis and therapy.
2016;
40
:
13-20
.
View Article PubMed Google Scholar -
I
Aditya,
V
Tat,
A
Sawana,
A
Mohamed,
R
Tuffner,
T
Mondal.
Use of Doppler velocimetry in diagnosis and prognosis of intrauterine growth restriction (IUGR): A Review. Journal of neonatal-perinatal medicine.
2016;
9
:
117-126
.
View Article Google Scholar -
RMY
Nomura,
JI
Niigaki,
FT
Horigome,
RPV
Francisco,
M
Zugaib.
Doppler velocimetry of the fetal middle cerebral artery and other parameters of fetal well-being in neonatal survival during pregnancies with placental insufficiency. Revista da Associação Médica Brasileira.
2013;
59
:
392-399
.
View Article PubMed Google Scholar -
R
Shahinaj,
N
Manoku,
E
Kroi,
I
Tasha.
The value of the middle cerebral to umbilical artery Doppler ratio in the prediction of neonatal outcome in patient with preeclampsia and gestational hypertension. Journal of prenatal medicine.
2010;
4
:
17
.
PubMed PMC Google Scholar -
AM
Dude,
LM
Yee.
Identifying Fetal Growth Disorders Using Ultrasonography in Women With Diabetes. Journal of Ultrasound in Medicine.
2017
.
PubMed Google Scholar -
MK
Tarzamni,
N
Nezami,
F
Gatreh-Samani,
S
Vahedinia,
M
Tarzamni.
Doppler waveform indices of fetal middle cerebral artery in normal 20 to 40 weeks pregnancies. Arch Iran Med.
2009;
12
:
29-34
.
PubMed Google Scholar -
EJ
Su.
Role of the fetoplacental endothelium in fetal growth restriction with abnormal umbilical artery Doppler velocimetry. American Journal of Obstetrics & Gynecology.
2015;
213
:
S123-S130
.
View Article PubMed PMC Google Scholar -
T
Nanthakomon,
B
Uerpairojkit.
Outcome of small-for-gestational-age fetuses according to umbilical artery Doppler: Is there any yield from additional middle cerebral artery Doppler?. The Journal of Maternal-Fetal & Neonatal Medicine.
2010;
23
:
900-905
.
View Article PubMed Google Scholar -
PN
Mena,
MC
Cubillos,
CJ
Toro,
CV
Zu-iga.
Intrauterine growth and biochemical levels in cord blood samples in infants less than 31 weeks gestation. Revista chilena de pediatria.
2016;
87
:
250-254
.
-
D
Sharma,
S
Shastri,
P
Sharma.
Intrauterine growth restriction: antenatal and postnatal aspects. Clinical Medicine Insights: Pediatrics.
2016;
10
:
CMPed-S40070
.
-
EF
Werner,
DA
Savitz,
TM
Janevic,
RM
Ehsanipoor,
SF
Thung,
EF
Funai,
HS
Lipkind.
Mode of delivery and neonatal outcomes in preterm, small-for-gestational-age newborns. Obstetrics and gynecology.
2012;
120
:
560
.
View Article PubMed PMC Google Scholar -
H
Dhand,
HK
Kansal,
A
Dave.
Middle cerebral artery Doppler indices better predictor for fetal outcome in IUGR. The Journal of Obstetrics and Gynecology of India.
2011;
61
:
166-171
.
View Article Google Scholar -
MI
Ibrahim,
MH
Akram,
M
Nafees.
SENSITIVITY AND SPECIFICITY OF PULSATILITY INDEX UMBILICAL ARTERY AND MIDDLE CEREBRAL ARTERY IN DETECTING INTRA UTERINE GROWTH RESTRICTION. Pakistan Armed Forces Medical Journal.
2014;
64
.
-
C
Monteith,
K
Flood,
S
Mullers,
J
Unterscheider,
F
Breathnach,
S
Daly,
MP
Geary,
MM
Kennelly,
FM
McAuliffe,
al
O'donoghue K et.
Evaluation of normalization of cerebro- placental ratio as a potential predictor for adverse outcome in SGA fetuses. American Journal of Obstetrics & Gynecology.
2017;
216
:
285-e1
.
View Article PubMed Google Scholar -
F
Cunningham,
K
Leveno,
S
Bloom,
CY
Spong,
J
Dashe.
Williams obstetrics, 24e. Mcgraw-hill.
2014
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 5 (2018)
Page No.: 2296-2304
Published on: 2018-05-21
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 16437 times
- Download PDF downloaded - 1924 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress