Abstract
Background: Lung Cancer (LC) is one of the most common cancers in the international arena. The aim of this study was to investigate the geographical distribution of LC incidence and mortality in the world in 2012, as well as the trend of incidence and mortality of LC during 1975 to 2010 based on the gender.
Methods: In the present study, we extracted the information on the incidence and mortality of LC in 184 countries from the International Agency for Research on Cancer (IARC) (Project GLOBOCAN, 2012). The present study categorized and presented the information on the Age-Standardized Incidence Rate (ASIR) and Age Standardized Mortality Rate (ASMR) of LC based on the continents, world regions based on the development level and Human Development Index (HDI). ASIR and ASMR of LC expressed per 100,000 people.
Results: The highest ASIR and ASMR of LC occurred in North America (ASIR=38.3 and ASMR=28.6), more developed regions (ASIR=30.8 and ASMR=24.2), and the WPRO region of the WHO (ASIR=32.8 and ASMR=28.5), and those regions with very high HDI (ASIR=31 and ASMR=23.9). Furthermore, the lowest ASIR and ASMR of LC occurred in Africa (ASIR=5 and ASMR=4.5), the less developed regions (ASIR=20 and ASMR=18), the AFRO region (ASIR=3.9 and ASMR=3.5), and regions with low HDI (ASIR=5.4 and ASMR=4.8).
Conclusion: The highest ASIR and ASMR of LC occurred in North America, more developed regions, and the WPRO region of the WHO, and those regions with very high HDI. Most regions of the world had decreasing incidence and mortality of LC in men and increasing trend in women.
Introduction
Despite the advances made in the field of prevention and control of communicable diseases over the last decade, the incidence and prevalence of Non-communicable diseases has increased dramatically and in most countries after the illness Cardiovascular disease is the second most common cause of death [1]–[5]. Lung Cancer (LC) is one of the most common cancers in the international arena. This disease is the most important cause of mortality from cancer, so that 20% of the cancer deaths are specifically due to the LC. 17% of men and 9% of women, who are diagnosed as the newly cases of cancer peer year, have LC [6].
Cigarette smoking is one of the most important risk factors for LC. Despite the descending prevalence of cigarette smoking in the United States since the 1950s, LC is now known as one of cancers with very high incidence, so that LC has the highest number of new cases after Prostate cancer in men and after breast cancer in women [7][8]. In the United States, different studies have shown a significant disparity in the incidence of LC in different races with an incidence of 50% in African Americans men higher than white males [7][8]. In another study on the incidence of disease in the United States, it was found that the incidence of LC was ascending in men until 1980 and in women up to 2007, but it was descending since then [8]. In the United States, LC accounts for a significant proportion of mortality from cancer, so that about 27 out of 100 deaths from cancer occurred due to LC [9].
The five-year survival rate for LC is very low, so that the 5-year survival rate of this cancer was equal to 12.3% in 1970 and 16.3% in 2007. However, the survival rate of this disease is highly associated with the diagnosis stage in such a way that the 5-year survival rate of this disease was equal to 52% in patients, whose diseases were at the local stages, and 24% in patients with diseases at the regional stage, while the 5-year survival rate for patients was equal to 4% in those whose diseases were at distant stage. However, there are other factors that affect the survival rate of disease, so that the survival rate is lower in males than females, in the elderly than adults, and in the African American race than other races [10][11].
As the burden of this disease on public health is so much, and the changes in its risk factors such as smoking is very different in the world, and due to the population growth and the increasing proportion of older people in societies, it is essential to perform the continuous and accurate epidemiology studies of this disease [12]. The incidence and mortality of LC are properly and regularly monitored in developed countries [13]–[16] however, there are not enough numbers of studies on the disparity in the distribution of incidence and mortality of this cancer based on different geographical or Socio-Economic indexes for areas at the national and international level [17]. Therefore, there are not identified geographical disparities caused by this disease at the global level. The aim of this study was to investigate the geographical distribution of LC incidence and mortality based on the development level, WHO classification, human development index levels and the world’s continents in 2012, as well as the trend of incidence and mortality of LC during 1975 to 2010 based on the gender in the worldwide.
Methods
In the present study, we extracted the information on the incidence and mortality of LC in 184 countries from the International Agency for Research on Cancer (IARC) (Project GLOBOCAN, 2012). GLOBOCAN is a database on various types of cancers and it is created by the World Health Organization. It covers information on the number, raw rates, and age standardization of cancer incidence, prevalence and mortality for different regions and countries. Currently, the available data in GLOBOCAN is known as one of the newest international database on the cancer. Based on the data of GLOBOCAN project, it is possible to investigate and compare the incidence and mortality of cancer based on the type of cancer, age and gender groups for different regions of the world.
The present study categorized and presented the information on the Age-Standardized Incidence Rate (ASIR) and Age Standardized Mortality Rate (ASMR) of LC based on the continents (Africa, Latin America and Caribbean, Northern America, Europe, Oceania, Asia), world regions based on the development level (More developed regions and Less developed regions) and human development index (very high, high, medium, or low).
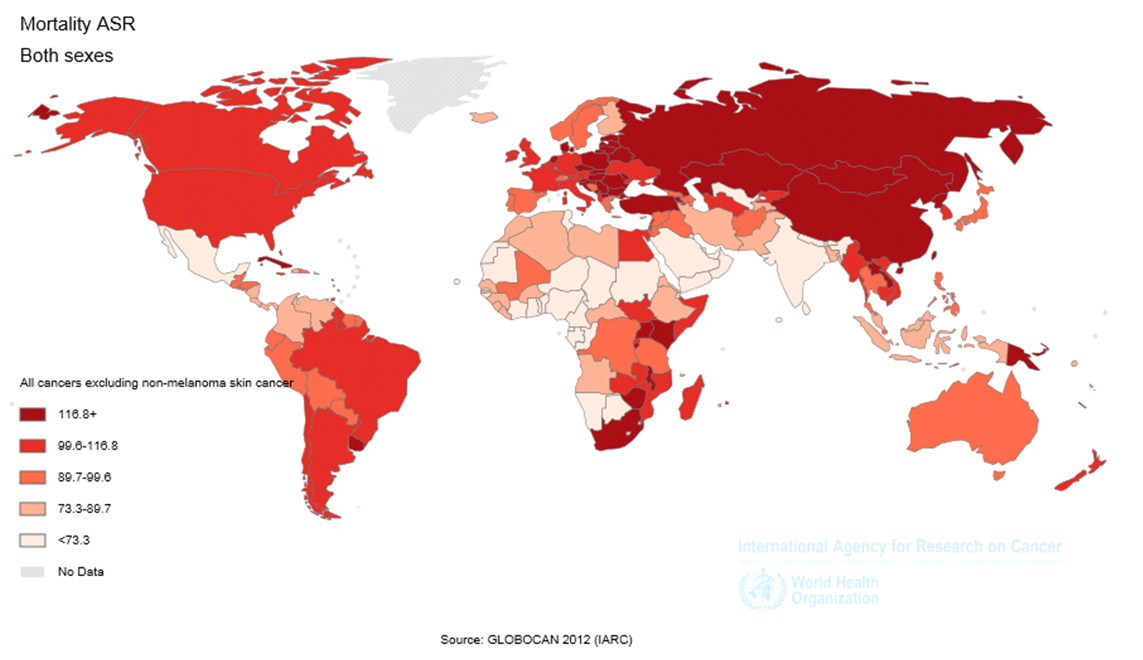
We provided the information about the incidence and mortality of LC based on the number, raw rates and the Age-Standardized rates in 2012. We also expressed raw and standardized rates of incidence and mortality per 100,000 people. Geographical Distribution Map was prepared for the ASIR and ASMR of this disease based on the age-specific rates. Detailed descriptions of applied methods are presented in previous reports [18]–[21].
Results
The ASIR and ASMR of LC in the world
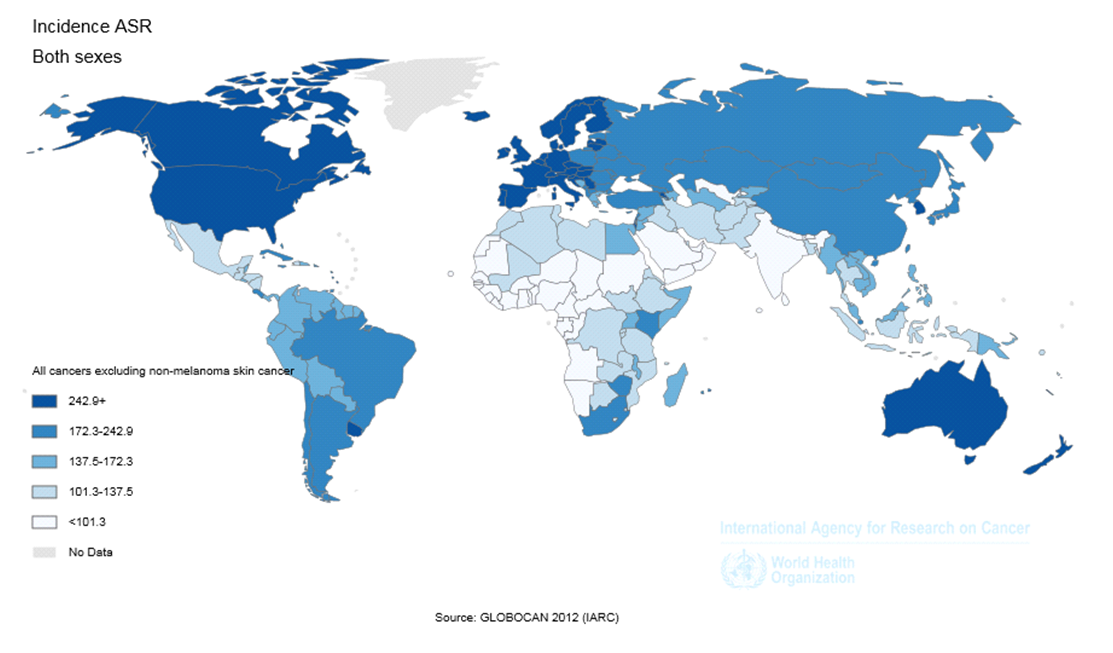
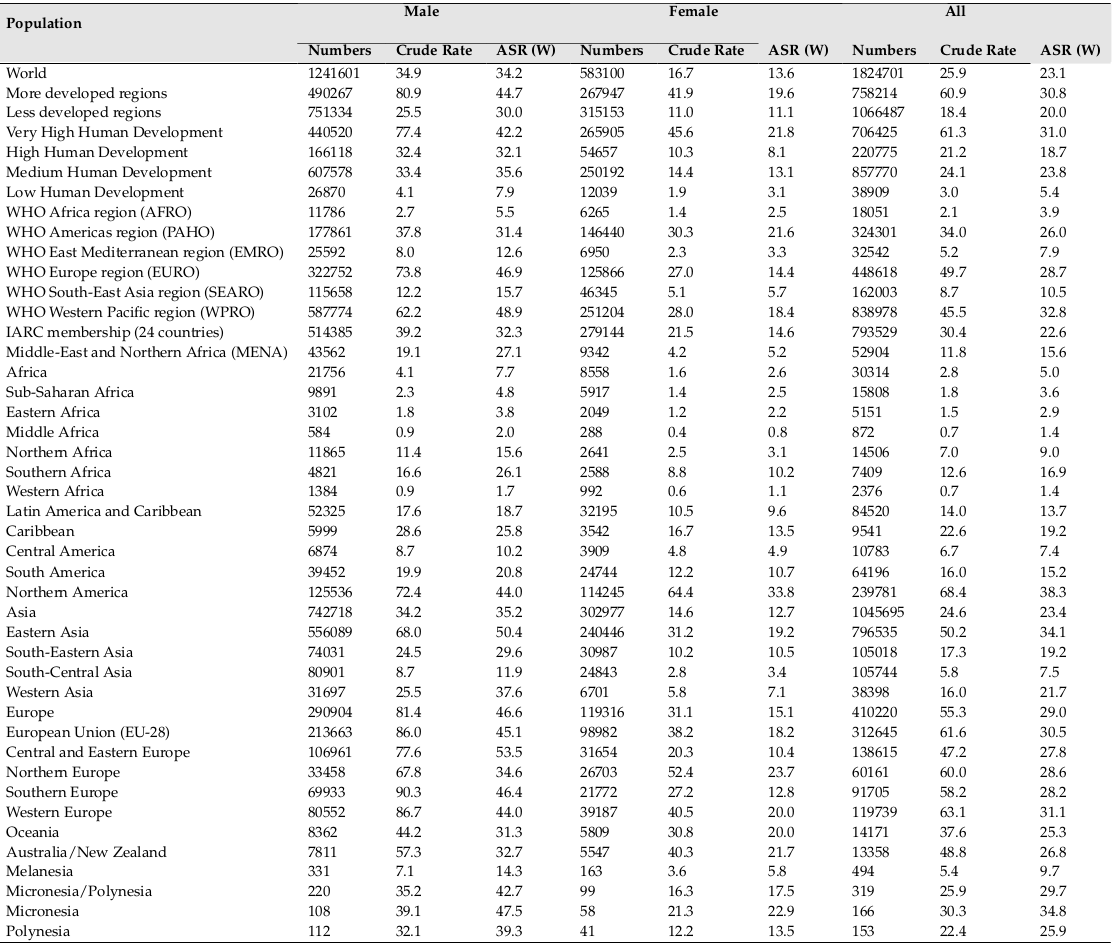
In 2012, 1824701 new cases of LC were diagnosed worldwide. From this number, 1241601 (68.04%) cases occurred in men and 583100 (31.96%) in women. In general, the ASIR of LC was equal to 23.1 (34.2 in men and 13.6 in women). The sex ratio (male to female) of the newly diagnosed LC was equal to 2.13 ( Figure 1 ).
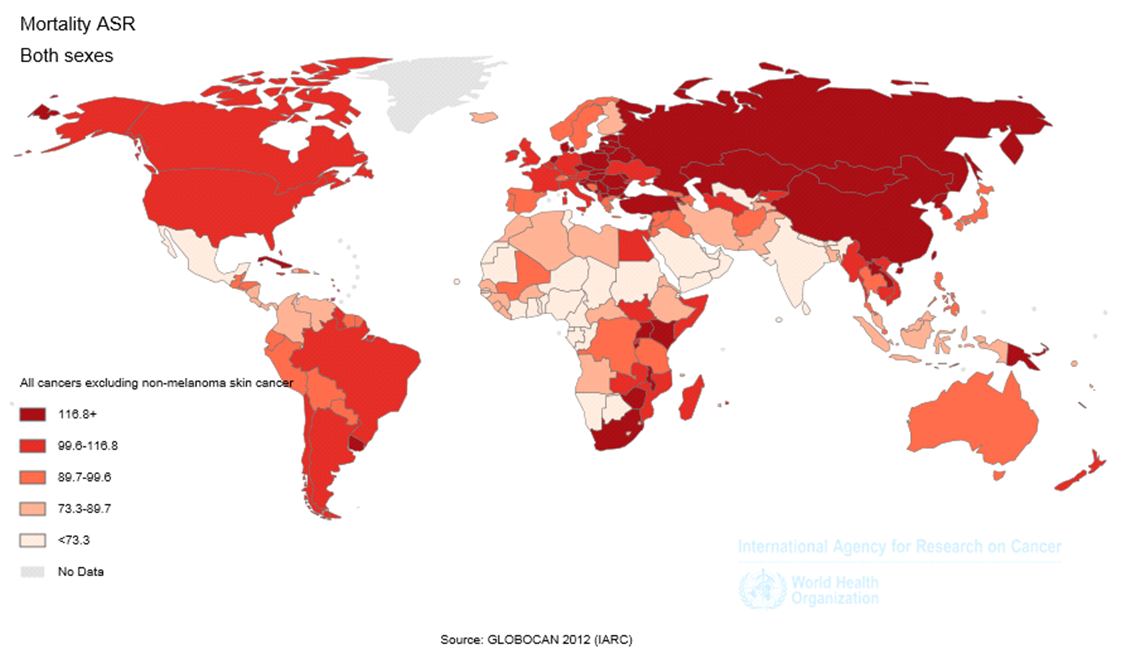
There were also 1589925 deaths from LC during this year. From this number, 1098702 (69.1%) cases occurred in men and 491223 (30.9%) in women. In general, the ASMR of LC was equal to 19.7 (30 were in men and 11.1 in women). The sex ratio of the mortality of LC was equal to 2.24 ( Figure 2 ).
The ASIR and ASMR of LC based on the continents
The ASIR of LC was equal to 38.3 (44 in men, and 33.8 in women) in North America, 29 (46.2 in men, and 15.1 in women) in Europe, 25.3 (31.3 in men and 20 in women) in Oceania, 23.4 (35.2 in men and 12.7 in women) in Asia, 13.7 (18.7 in men and 9.6 in women) in Latin America, and 5 (7.7 in men and 2.6 in women) in Africa. From the total number of this disease in the world, the highest proportion occurred in Asia and the lowest proportion in Oceania, so that 57.3% of cases occurred in Asia, 22.5% in Europe, 13.2% in North America, 4.7% in Latin America, 1.6% in Africa and 0.7% in Oceania ( Table 1 and Figure 1 , Figure 2 and Figure 3 ).
The ASMR of LC was equal to 28.6 (34.8 in men, and 23.5 in women) in North America, 24 (39.8 in men, and 11.8 in women) in Europe, 20.7 (31.6 in men and 11 in women) in Asia, 18.3 (23.1 in men and 14.1 in women) in Oceania, 12 (16.7 in men and 8.1 in women) in Latin America, and 4.5 (7 in men and 2.4 in women) in Africa. From the total number of deaths of LC in the world, the highest proportion occurred in Asia and the lowest proportion in Oceania, so that 58.8% of cases occurred in Asia, 22.3% in Europe, 11.8% in North America, 4.7% in Latin America, 1.7% in Africa and 0.7% in Oceania ( Table 2 and Figure 3 ).
The ASIR and ASMR of LC based on the development level
The ASIR of LC was equal to 30.8 (44.7 in men, and 19.6 in women) in more developed countries, and 20 (30 in men, and 11.1 in women) in less developed regions. 58.4% of cases occurred in more developed countries and 41.6% in less developed regions.
The mortality of LC was equal to 24.2 (36.8 in men, and 14.3 in women) in more developed regions, and 18 (27.2 in men, and 9.8 in women) in less developed regions. 39.4% of cases occurred in more developed regions and 60.6% in less developed regions.
The ASIR and ASMR of LC according to the WHO classification
The ASIR of LC was equal to 32.8 (48.9 in men, and 18.4 in women) in WPRO, 28.7 (46.9 in men, and 14.4 in women) in EURO, 26 (31.4 in men and 21.6 in women) in PAHO, 10.5 (15.7 in men and 5.7 in women) in SEARO, 7.9 (12.7 in men and 3.3 in women) in EMRO, and 3.9 (5.5 in men and
2.5 in women) in AFRO. From the total incidence of this disease, 46% occurred in WPRO, 24.6% in EURO, 17.7% in PAHO, 8.9% in SEARO, 1.8% in EMRO, and 1% in AFRO ( Table 1 ).
The ASMR of LC was equal to 28.5 (43.3 in men, and 15.4 in women) in WPRO, 24 (40.3 in men, and 11.3 in women) in EURO, 20.4 (25.9 in men and 15.9 in women) in PAHO, 9.5 (14.2 in men and 5.2 in women) in SEARO, 7.1 (11.3 in men and 2. in women) in EMRO, and 3.5 (5 in men and
2.3 in women) in AFRO. From the total mortality of this disease, 47.1% occurred in WPRO, 24.4% in EURO, 16.5% in PAHO, 9.2% in SEARO, 1.8% in EMRO, and 1% in AFRO ( Table 2 ).
The ASIR and ASMR of LC according to the levels of Human Development Index (HDI)
The ASIR of LC was equal to 31 (42.2 in men, and 21.8 in women) in regions with very high HDI, 18.7 (32.1 in men, and 8.1 in women) in regions with high HDI, 23.8 (35.6 in men and 13.1 in women) in regions with medium HDI, and 5.4 (7.9 in men and 3.1 in women) in regions with low HDI. From the total incidence of this disease, 47% occurred in regions with medium HDI, 38.7% in regions with very high HDI, 12.1% in regions with high HDI, and 2.2% in regions with low HDI ( Table 1 ).
The ASMR of LC was equal to 23.9 (34.1 in men, and 15.6 in women) in regions with very high HDI, 16.4 (28.6 in men, and 6.9 in women) in regions with high HDI, 21.6 (32.6 in men and 11.7 in women) in regions with medium HDI, and 4.8 (7.1 in men and 2.8 in women) in regions with low HDI. From the total mortality of this disease, 49.1% occurred in regions with medium HDI, 36.4% in regions with very high HDI, 12.3% in regions with high HDI, and 2.2% in regions with low HDI ( Table 2 ).
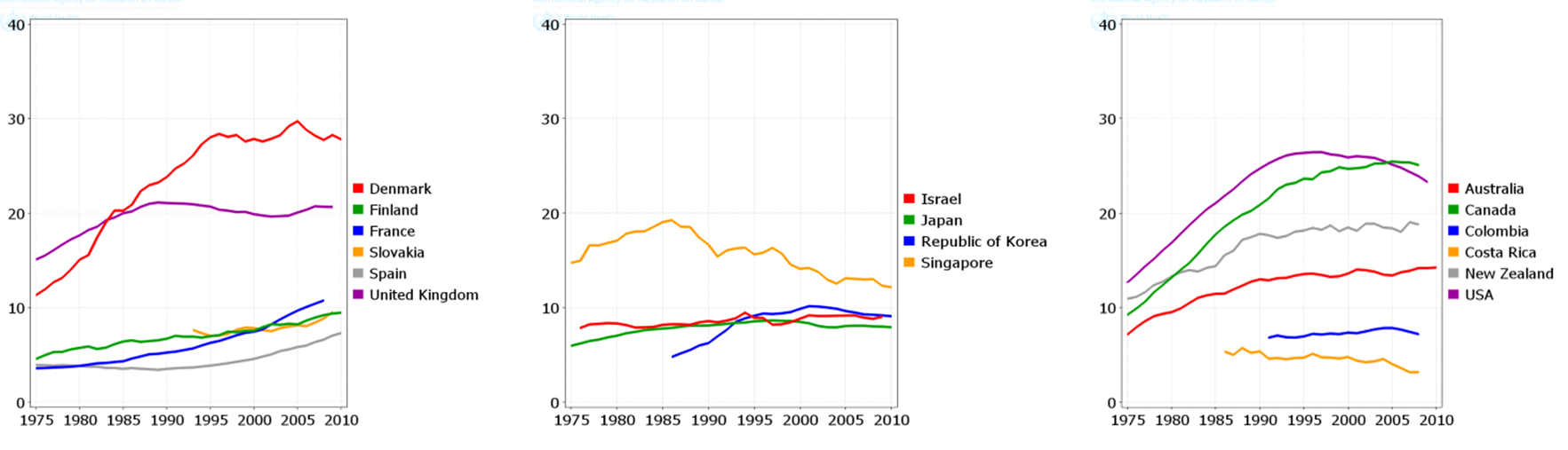
The trend of LC ASIR and ASMR during 1950 to 2010
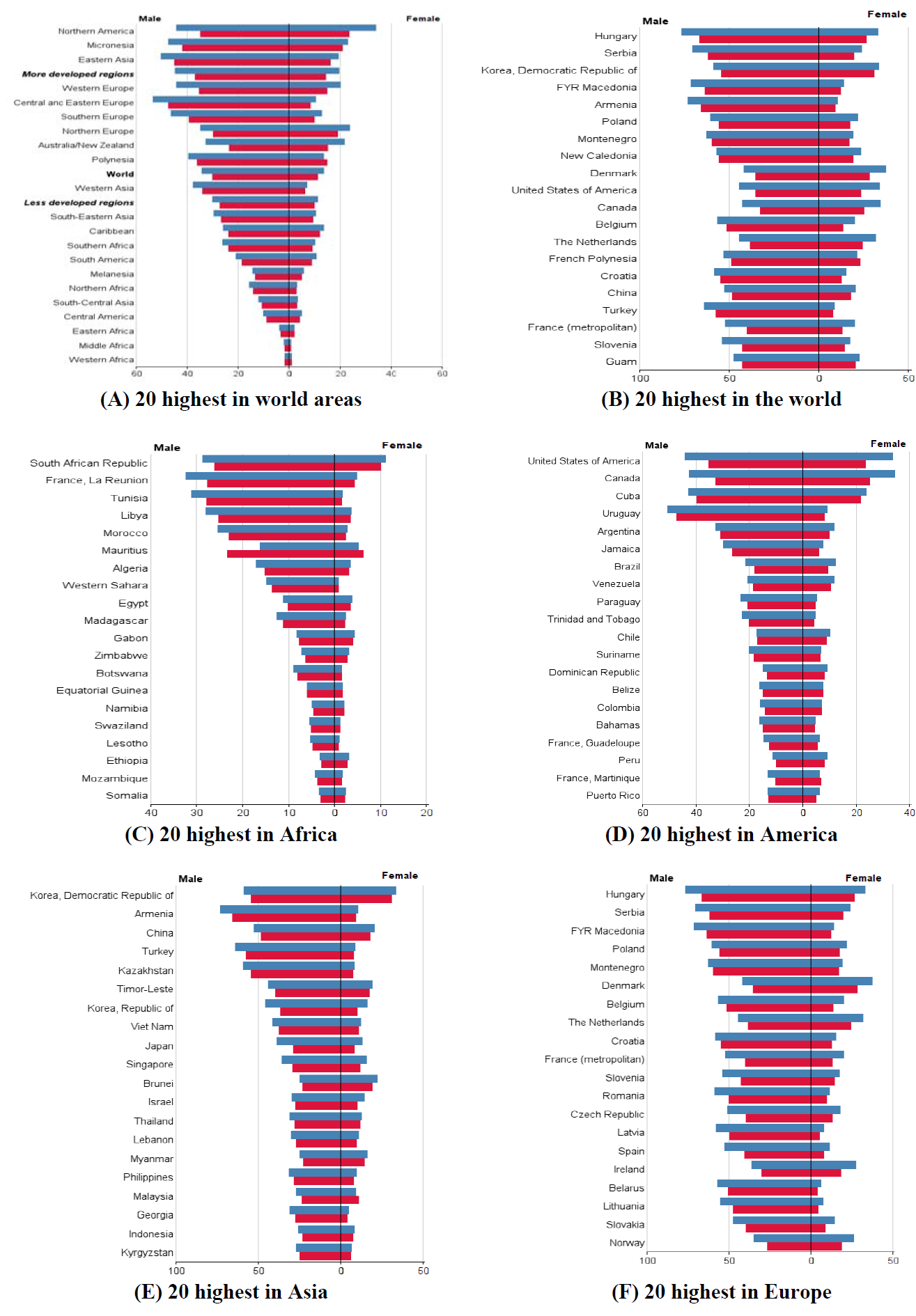
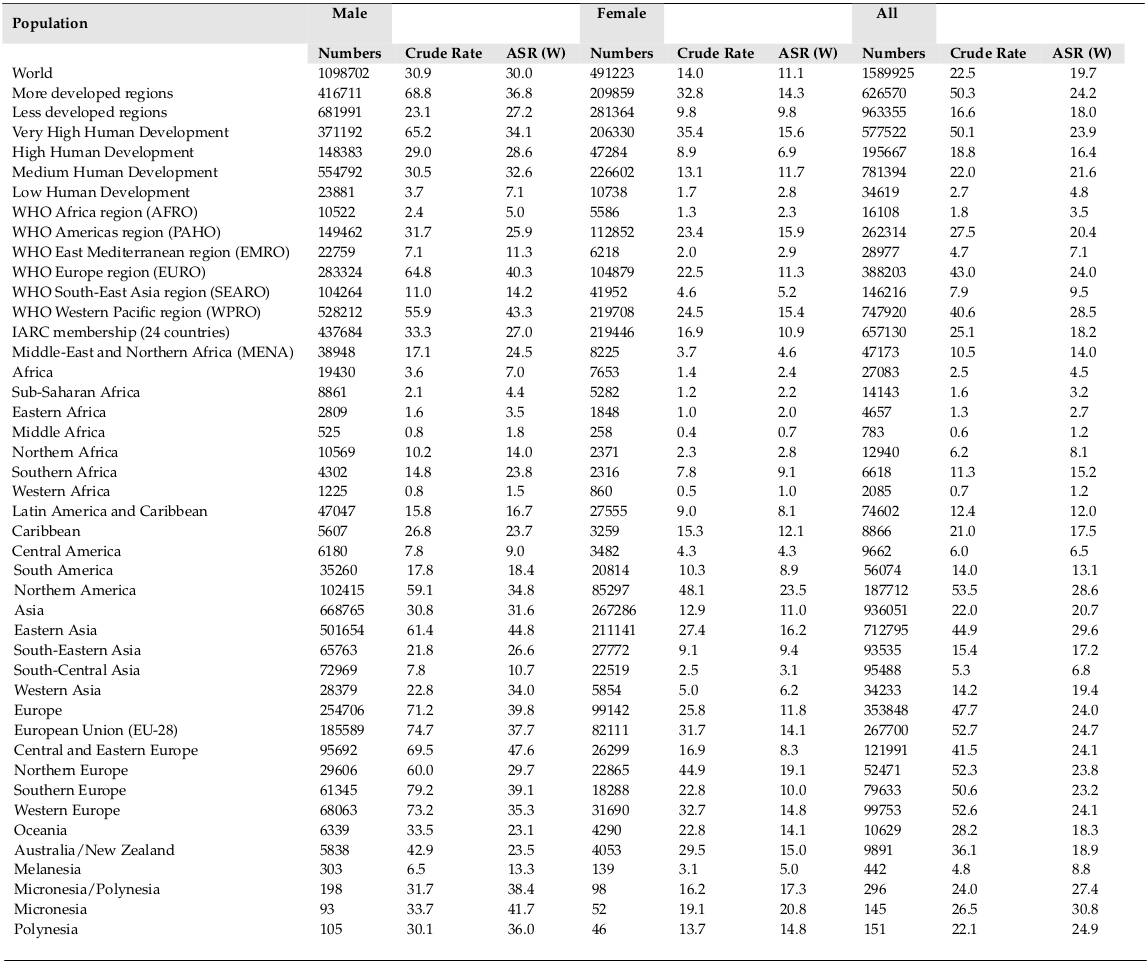
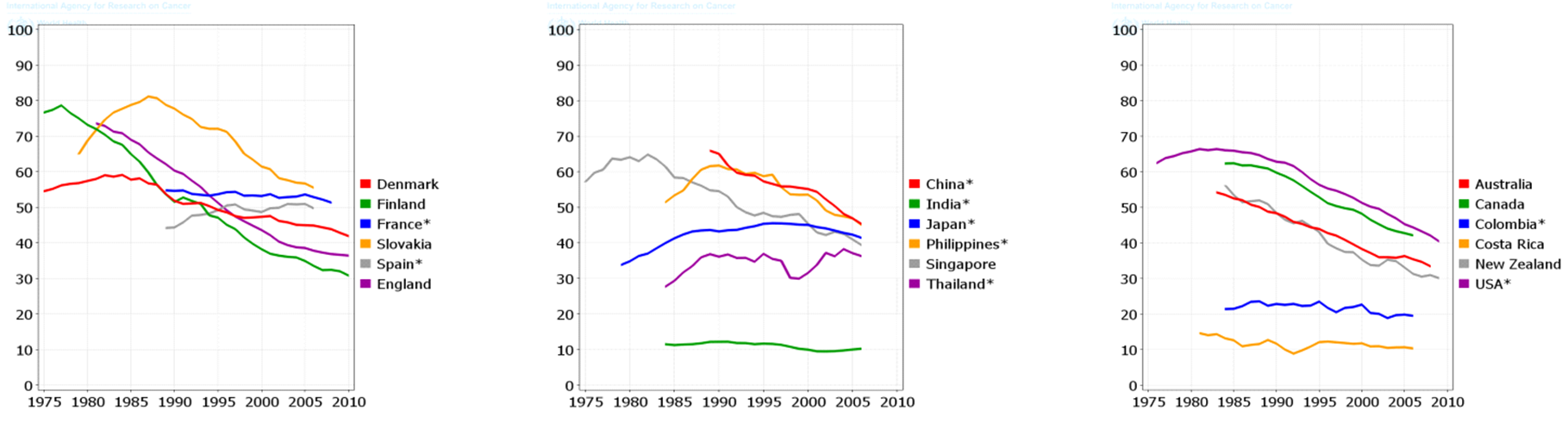
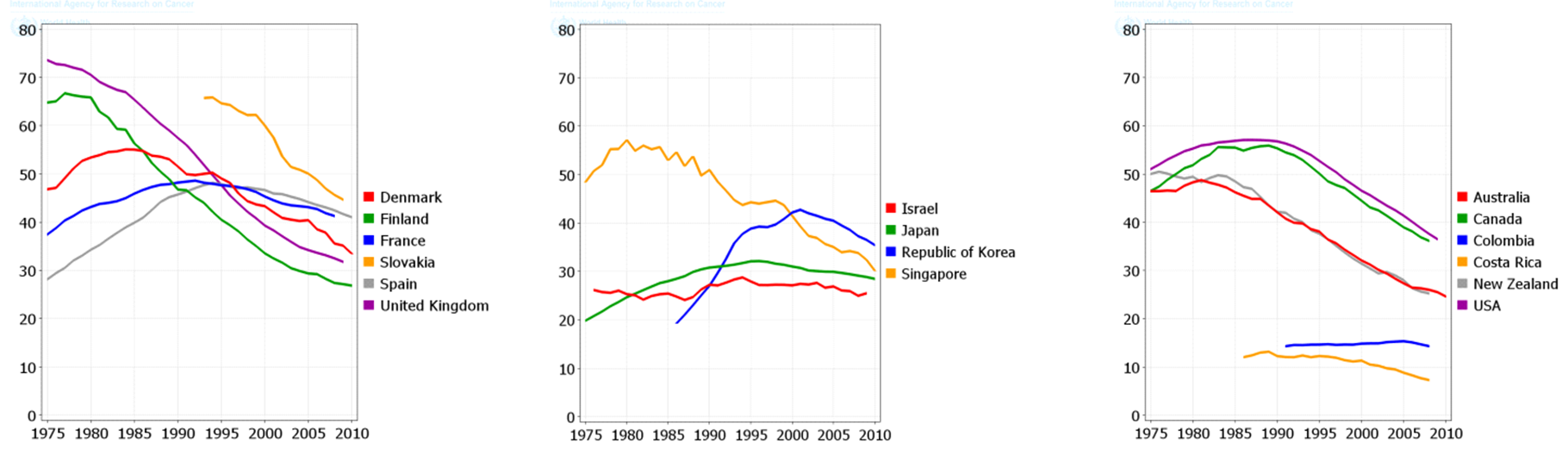
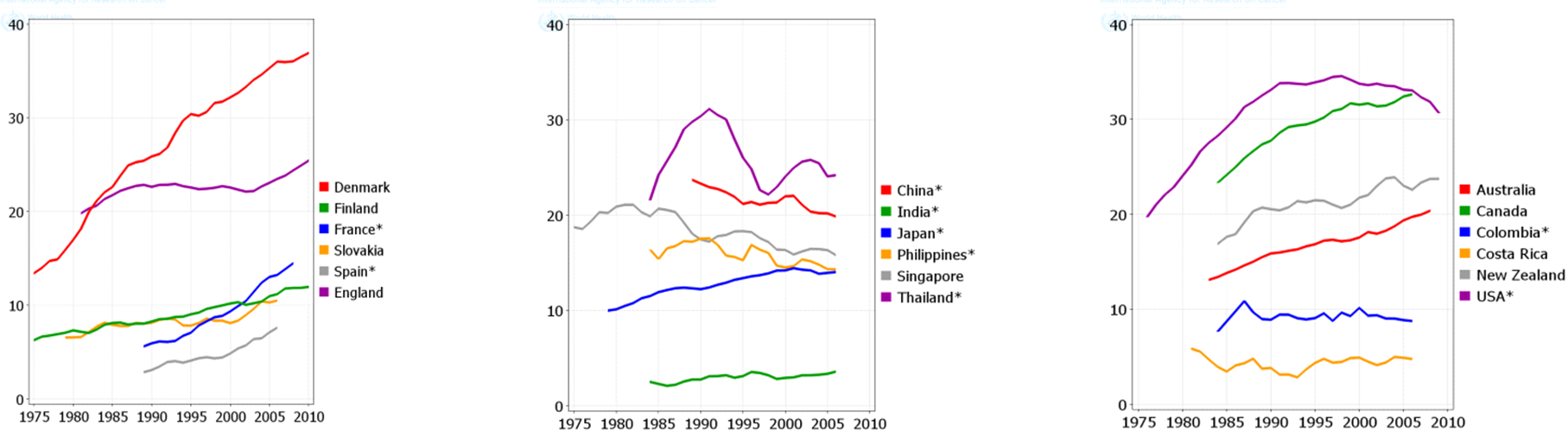
The total trend of ASIR and ASMR of LC was descending in men during 1975 to 2010, while it was quite different in women and it was ascending during this period. Figure 4 , Figure 5 , Figure 6 and Figure 7 show the trend of incidence and mortality of LC in men and women in some countries during 1975 to 2010.
Discussion
The present study aimed to investigate the distribution of LC incidence and mortality worldwide based on the WHO classification, the world’s continents, development level, and the HDI level based on the gender in 2012. It also studied the trend of incidence and mortality of LC based on the gender for some selected countries during 1975 to 2010. In 2012, there were 1824701 new cases of LC in worldwide, of which 68.04% were in men and 31.96% in women. This year also had 1589925 deaths from this disease of which 69.1% were in men and 30.9% in women. The highest ASIR and ASMR of LC occurred in North America, more developed regions, and the WPRO region of the WHO, and regions with very high HDI. Furthermore, the lowest ASIR and ASMR of LC occurred in Africa, the less developed regions, the AFRO region, and regions with low HDI. The ASIR and ASMR of LC was descending in men, but ascending in women in most of the world’s countries from 1975 to 2010.
The highest ASIR and ASMR of LC occurred in areas with very high HDI, but the lowest rate was seen in areas with low HDI. Similar results with the present research were found in a study by Pakzad et al on the relationship between the HDI and the ASIR and ASMR of LC in Asian countries. According to their study, an increase in the HDI led to the increased ASIR and ASMR of LC, so that the correlation was equal to 0.345 between the HDI and the ASIR, and equal to 0.289 with ASMR, that it was statistically significant [17]. High life expectancy and average life span were the main reasons for the high ASIR and ASMR of LC in regions with very high HDI. These regions may also have much higher prevalence of smoking than other regions of the world. For instance, the annual cigarette consumption per capita was increased more than 70 times (from 54 to 4,000 cigarettes) in the United States from 1880 to 1970 (12). On the other hand, there might be the under estimate of incidence and mortality number of cancer cases in regions with low HDI due to the existing problems with diagnosis of the disease as well as problems with the registration of cancer cases, and thus the actual cases of this disease were higher than the diagnosed and recorded cases. These problems have also been observed in other cancer studies [19][20][22]-[28].
In this study, the incidence and mortality number of LC in men was twice as high as in women, so that 68% of incidence and 69% of mortality of LC occurred in men. Similar results were observed in a study by Pakzad et al in Asian countries, to that 71.13% of new cases and 71.45% of deaths occurred in men in Asia during 2012 [17]. Probably, the main reason for the disparity in the incidence and mortality of LC among men and women is the disparity in their exposure to the risk factors of the disease.
The geographical distribution of LC was largely associated with the prevalence of cigarette smoking, so that in Kentucky State of the United States, where the prevalence of cigarette smoking was equal to 30%, the ASIR of LC was 125.9 in men, and 80.3 in women per 100,000 people, while in California, where the prevalence of cigarette smoking was 14%, the ASIR of LC was 60.4 in men and 44.4% in women per 100,000 people [29]. During 1973 to 2007, the incidence of LC was steadily decreasing in white women in California, while no decreasing trend of mortality was seen in women in Midwestern and Southern States, indicating the effectiveness of control programs in reducing the incidence of cigarette smoking in California State [30].
The maximum prevalence of cigarette smoking in men has been two decades earlier than women; hence, it is expected that the epidemic of this LC will begin in women at a time interval after men. The incidence of LC in men has been descending over the past few decades, while the incidence of this disease is still ascending in women [30]. Despite the fact that the number of men, who annually die from LC, is much higher than women, it is expected that over the years to come the disparity in mortality rates between both genders will become minimal [10].
Cigarette is thus one of the most important factors affecting the incidence of LC worldwide. Furthermore, due to the high cost and limited effectiveness of screening methods and therapeutic measures in LC, cigarette is one of the key factors in the death from LC [31]. Therefore, effective measures can be taken to reduce the incidence of cigarette smoking to reduce the burden of LC in the global arena especially in the African regions and some Asian countries with limited access to screening and treatment programs [32]. In areas such as Australia, California and Hong Kong, which have begun the cigarette prevention programs, the incidence of LC is expected to reduce significantly especially in men. Since there is a long latency interval between smoking and occurrence of LC, the incidence of LC will significantly increase in countries like China, where there is not any adequate program to reduce the prevalence of cigarette smoking, in the upcoming decades [33].
Therefore, governments should adopt appropriate planning and policies to prevent LC. In addition to reduction of the prevalence of smoking, they should also reduce the exposure to other well-known risk factors such as Asbestos, Radon, air pollution, occupational carcinogens and second-hand smoke [34][35].
This study, has been conducted using data of GLOBOCAN project of World Health Organization(WHO) in 2012. The aim of the GLOBOCAN project is to provide contemporary estimates of the incidence, mortality and prevalence from major types of cancer, at national level, for 184 countries of the world. Although data from the GLOBOCAN project is currently the most comprehensive source of information on the distribution of cancers worldwide. Nevertheless, one of the limitations of this study is the use of data of 2012, due to the lack of newer data in the global level. Also, the quality of collected cancer data in the GLOBOCAN project is not similar in all countries especially those with medium or low HDI, so that the estimates of some countries are based on the recorded cases of cancer in limited areas or based on estimates from neighboring countries [36].
Conclusion
The highest ASIR and ASMR of LC occurred in North America, more developed regions, and the WPRO region of the WHO, and those regions with very high HDI. Furthermore, the lowest ASIR and ASMR of LC occurred in Africa, the less developed regions, the AFRO region, and regions with low human development indexes. Most regions of the world had decreasing incidence and mortality of LC in men and increasing trend in women.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
AFRO: WHO Africa region, ASIR: Age- Standardized Incidence Rate, ASMR: Age- Standardized Mortality Rate, EMRO: WHO East Mediterranean region, EURO: WHO Europe region, HDI: Human Development Index, IARC: International Agency for Research on Cancer, PAHO: WHO Americas region, SEARO: WHO South-East Asia region, WHO: World Health Organization, WPRO: WHO Western Pacific region
Ethics approval and consent to participate
Not to be applied
Competing interests
The authors declare that they have no conflicts of interest.
Funding
None
Authors’ contributions
All authors contributed to the design of the research. MM, HS, MA, FAB and AMH extracted the data and summarized it. All authors drafted the first version. HSG, AS, AMH and KAB edited the first draft. All authors reviewed, commented and approved the final draft.
References
-
ABM
Hafshejani,
H
Baradaran,
N
Sarrafzadegan,
MA
Lari,
A
Ramezani,
SH
Hosseini,
FA
Hafshejani.
Predicting factors of short-term survival in patients with acute myocardial infarction in Isfahan using a cox regression model. Iranian Journal of Epidemiology.
2012a;
8
:
39-47
.
-
AM
Hafshejani,
N
Sarrafzadegan,
A
Moghaddam,
HR
Baradaran,
S
Hosseini,
MA
Lari.
Gender Difference in Determinants of Short-Term Survival of Patients with Acute Myocardial Infarction in Isfahan, Iran. Journal of Isfahan Medical School.
2012b;
30
.
-
A
Mohammadian-Hafshejani,
N
Sarrafzadegan,
S
Hosseini,
HR
Baradaran,
H
Roohafza,
M
Sadeghi,
M
Asadi-Lari.
Seasonal pattern in admissions and mortality from acute myocardial infarction in elderly patients in Isfahan, Iran. ARYA atherosclerosis.
2014;
10
:
46
.
PubMed PMC Google Scholar -
T
Vos,
RM
Barber,
B
Bell,
A
Bertozzi-Villa,
S
Biryukov,
I
Bolliger,
F
Charlson,
A
Davis,
L
Degenhardt,
D
Dicker.
Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet.
2015;
386
:
743-800
.
View Article Google Scholar -
F
Hu,
B
Hu,
R
Chen,
Y
Ma,
L
Niu,
X
Qin,
Z
Hu.
A systematic review of social capital and chronic non-communicable diseases. Bioscience trends.
2014;
8
:
290-296
.
View Article PubMed Google Scholar -
LA
Torre,
F
Bray,
RL
Siegel,
J
Ferlay,
J
Lortet-Tieulent,
A
Jemal.
Global cancer statistics, 2012. CA: a cancer journal for clinicians.
2015;
65
:
87-108
.
View Article PubMed Google Scholar -
J
Lortet-Tieulent,
I
Soerjomataram,
J
Ferlay,
M
Rutherford,
E
Weiderpass,
F
Bray.
International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung cancer.
2014;
84
:
13-22
.
View Article Google Scholar -
R
Meza,
C
Meernik,
J
Jeon,
ML
Cote.
Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PloS one.
2015;
10
:
e0121323
.
View Article PubMed PMC Google Scholar -
R
Siegel,
D
Naishadham,
A
Jemal.
Cancer statistics, 2013. CA: a cancer journal for clinicians.
2013;
63
:
11-30
.
View Article PubMed Google Scholar -
AJ
Alberg,
MV
Brock,
JG
Ford,
JM
Samet,
SD
Spivack.
Epidemiology of lung cancer: Diagnosis and management of lung cancer: American College of Chest Physicians evidence- based clinical practice guidelines. Chest.
2013;
143
:
e1S-e29S
.
View Article PubMed PMC Google Scholar -
LA
Ries,
D
Harkins,
M
Krapcho,
A
Mariotto,
BA
Miller,
EJ
Feuer,
LX
Clegg,
MP
Eisner,
MJ
Horner,
N
Howlader.
SEER cancer statistics review 1975-2003. 2006
.
-
TYD
Cheng,
SM
Cramb,
PD
Baade,
DR
Youlden,
C
Nwogu,
ME
Reid.
The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. Journal of Thoracic Oncology.
2016;
11
:
1653-1671
.
View Article PubMed PMC Google Scholar -
C
Bosetti,
M
Malvezzi,
T
Rosso,
P
Bertuccio,
S
Gallus,
L
Chatenoud,
F
Levi,
E
Negri,
CL
Vecchia.
Lung cancer mortality in European women: trends and predictions. Lung Cancer.
2012;
78
:
171-178
.
View Article PubMed Google Scholar -
J
Ferlay,
E
Steliarova-Foucher,
J
Lortet-Tieulent,
S
Rosso,
JWW
Coebergh,
H
Comber,
D
Forman,
F
Bray.
Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer.
2013;
49
:
1374-1403
.
View Article PubMed Google Scholar -
JAGW
Blajer,
K
Mariusz.
The Inverse Simulation Study of Aircraft Flight Path Reconstruction. Transport.
2002;
XVII
:
103-107
.
-
DR
Lewis,
DP
Check,
NE
Caporaso,
WD
Travis,
SS
Devesa.
US lung cancer trends by histologic type. Cancer.
2014;
120
:
2883-2892
.
View Article PubMed PMC Google Scholar -
R
Pakzad,
A
Mohammadian-Hafshejani,
M
Ghoncheh,
I
Pakzad,
H
Salehiniya.
The incidence and mortality of lung cancer and their relationship to development in Asia. Translational lung cancer research.
2015;
4
:
763
.
PubMed PMC Google Scholar -
M
Arabsalmani,
A
Mohammadian-Hafshejani,
M
Ghoncheh,
F
Hadadian,
F
Towhidi,
K
Vafaee,
H
Salehiniya.
Incidence and mortality of kidney cancers, and human development index in Asia; a matter of concern. Journal of nephropathology.
2017;
6
:
30
.
View Article PubMed PMC Google Scholar -
M
Arabsalmani,
M
Mirzaei,
A
Soroush,
F
Towhidi,
H
Salehiniya.
Incidence and mortality of liver cancer and their relationship with the human development index in the world. Biomedical Research and Therapy.
2016;
3
:
800-807
.
View Article Google Scholar -
H
Rafiemanesh,
A
Mohammadian-Hafshejani,
M
Ghoncheh,
Z
Sepehri,
R
Shamlou,
H
Salehiniya,
F
Towhidi,
BR
Makhsosi.
Incidence and mortality of colorectal cancer and relationships with the human development index across the world. Asian Pacific journal of cancer prevention: APJCP.
2016;
17
:
2465-73
.
PubMed Google Scholar -
M
Shuja,
SI
Farsani,
H
Salehiniya,
S
Khazaei,
M
Mohammadian,
M
Aryaie,
P
Bagheri,
FA
Bakeshei,
A
Mohammadian-Hafshejani.
Assessment the association between liver cancer incidence and mortality rate with human development index in the European countries in 2012. Biomedical Research and Therapy.
2017;
4
:
1185-1197
.
View Article Google Scholar -
R
Pakzad,
A
Mohammadian-Hafshejani,
B
Khosravi,
S
Soltani,
I
Pakzad,
M
Mohammadian,
H
Salehiniya,
Z
Momenimovahed.
The incidence and mortality of esophageal cancer and their relationship to development in Asia. Annals of translational medicine.
2016;
4
.
-
S
Razi,
M
Ghoncheh,
A
Mohammadian-Hafshejani,
H
Aziznejhad,
M
Mohammadian,
H
Salehiniya.
The incidence and mortality of ovarian cancer and their relationship with the Human Development Index in Asia. ecancermedicalscience.
2016;
10
.
-
H
Rafiemanesh,
M
Mehtarpoor,
A
Mohammadian-Hafshejani,
H
Salehiniya,
M
Enayatrad,
S
Khazaei.
Cancer epidemiology and trends in Sistan and Baluchestan province, Iran. Medical journal of the Islamic Republic of Iran.
2015;
29
:
254
.
PubMed PMC Google Scholar -
S
Razi,
M
Enayatrad,
A
Mohammadian-Hafshejani,
H
Salehiniya.
The epidemiology of skin cancer and its trend in Iran. International journal of preventive medicine.
2015;
6
.
-
S
Hassanipour-Azgomi,
A
Mohammadian-Hafshejani,
M
Ghoncheh,
F
Towhidi,
S
Jamehshorani,
H
Salehiniya.
Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate international.
2016;
4
:
118-124
.
View Article PubMed PMC Google Scholar -
H
Rafiemanesh,
F
Maleki,
A
Mohammadian-Hafshejani,
M
Salemi,
H
Salehiniya.
The trend in histological changes and the incidence of esophagus cancer in Iran (2003-2008). International journal of preventive medicine.
2016;
7
.
-
H
Salehiniya,
SG
Dashdebi,
H
Rafiemanesh,
A
Mohammadian-Hafshejani,
M
Enayatrad.
Time Trend Analysis of Cancer Incidence in Caspian Sea, 2004-2009: A Population-based Cancer Registries Study (northern Iran). Caspian journal of internal medicine.
2016;
7
:
25
.
PubMed PMC Google Scholar -
R
Siegel,
J
Ma,
Z
Zou,
A
Jemal.
Cancer statistics, 2014. CA: a cancer journal for clinicians.
2014;
64
:
9-29
.
View Article PubMed Google Scholar -
A
Jemal,
J
Ma,
PS
Rosenberg,
R
Siegel,
WF
Anderson.
Increasing lung cancer death rates among young women in southern and midwestern States. Journal of Clinical Oncology.
2012;
30
:
2739
.
View Article PubMed PMC Google Scholar -
M
Ezzati,
SJ
Henley,
AD
Lopez,
MJ
Thun.
Role of smoking in global and regional cancer epidemiology: current patterns and data needs. International journal of cancer.
2005;
116
:
963-971
.
View Article PubMed Google Scholar -
V
Winkler,
JJ
Ott,
M
Cowan,
H
Becher.
Smoking prevalence and its impacts on lung cancer mortality in Sub-Saharan Africa: an epidemiological study. Preventive medicine.
2013;
57
:
634-640
.
View Article PubMed Google Scholar -
ED
Paskett,
BM
Bernardo,
FR
Khuri.
Tobacco and China: the worst is yet to come. Cancer.
2015;
121
:
3052-3054
.
View Article PubMed Google Scholar -
JM
Laughlin.
An historical overview of radon and its progeny: applications and health effects. Radiation protection dosimetry.
2012;
152
:
2-8
.
View Article PubMed Google Scholar -
CM
Wong,
N
Vichit-Vadakan,
N
Vajanapoom,
B
Ostro,
TQ
Thach,
PY
Chau,
EK
Chan,
RY
Chung,
CQ
Ou,
L
Yang.
Part 5. Public health and air pollution in Asia (PAPA): a combined analysis of four studies of air pollution and mortality. Research report (Health Effects Institute).
2010;
:
377-418
.
PubMed Google Scholar -
J
Ferlay,
I
Soerjomataram,
R
Dikshit,
S
Eser,
C
Mathers,
M
Rebelo,
DM
Parkin,
D
Forman,
F
Bray.
Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer.
2015;
136
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 6 (2018)
Page No.: 2348-2364
Published on: 2018-06-23
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 12090 times
- Download PDF downloaded - 2341 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress