Abstract
BACKGROUND: Chronic urticaria (CU) is one of the common allergic diseases whose conventional treatments have failed to desirably manage it. Fumariavaillantii is used in Persian medicine to treat CU. The anti-inflammatory and anti-histaminic effects of chemical components of Fumaria such as fumaric acid and caffeic acid were confirmed. Dimethyl fumarate reduces the pro- inflammatory contribution and monomethyl fumarate can increase IL-4, an antiinflammatory interleukin, or can decrease IFN- , an inflammatory factor. The current study assesses the efficacy and tolerability of Fumaria vaillantii versus cetirizine in the management of CU.
METHODS: The formulation and standardization of Fumaria syrup were done in Tehran University of Medical Sciences. Patients were randomized to twice- daily treatment with Fumaria syrup or cetirizine syrup (n=39 in each group) for four weeks. The efficacy assessment included Urticaria Activity Score (UAS) and Chronic Urticaria Quality of Life Questionnaire (CUQ2oL) and the safety evaluations included Common Terminology Criteria for Adverse Events Questionnaire.
RESULTS: The fumaric acid content in 5 ml of Fumaria syrup was calculated to be 0.12 mg. The results of clinical trial showed that UAS was significantly higher in the Fumaria group than in the cetirizine group, after the first week of follow-up (p<0.001), but no significant difference was demonstrated between the two groups on week 4 (p=0.57). One month after the research was finished, the UAS score of the cetirizine group was significantly higher than that of the Fumaria group (p<0.001). After finishing the interventions, difference of CU-Q2oL was not significant between the two groups; however, the QOL score was significantly lower in the Fumaria group (p<0.001) at 8th week. About adverse events, the incidence of somnolence in the Fumaria group was significantly lower than in the cetirizine group (p<0.001).
CONCLUSIONS: Fumaria vaillantii demonstrated its effects on CU later than cetirizine, but led to more permanent effects, better quality of life, and lower incidence of adverse events as compared to cetirizine. More clinical trials with higher populations are needed to achieve more conclusive results.
Abstract
BACKGROUND: Chronic urticaria (CU) is one of the common allergic diseases whose conventional treatments have failed to desirably manage it. Fumariavaillantii is used in Persian medicine to treat CU. The anti-inflammatory and anti-histaminic effects of chemical components of Fumaria such as fumaric acid and caffeic acid were confirmed. Dimethyl fumarate reduces the pro- inflammatory contribution and monomethyl fumarate can increase IL-4, an anti- inflammatory interleukin, or can decrease IFN- γ, an inflammatory factor. The current study assesses the efficacy and tolerability of Fumaria vaillantii versus cetirizine in the management of CU. METHODS: The formulation and standardization of Fumaria syrup were done in Tehran University of Medical Sciences. Patients were randomized to twice- daily treatment with Fumaria syrup or cetirizine syrup (n=39 in each group) for four weeks. The efficacy assessment included Urticaria Activity Score (UAS) and Chronic Urticaria Quality of Life Questionnaire (CU- Q2oL) and the safety evaluations included Common Terminology Criteria for Adverse Events Questionnaire. RESULTS: The fumaric acid content in 5 ml of Fumaria syrup was calculated to be 0.12 mg. The results of clinical trial showed that UAS was significantly higher in the Fumaria group than in the cetirizine group, after the first week of follow-up (p<0.001), but no significant difference was demonstrated between the two groups on week 4 (p=0.57). One month after the research was finished, the UAS score of the cetirizine group was significantly higher than that of the Fumaria group (p<0.001). After finishing the interventions, difference of CU-Q2oL was not significant between the two groups; however, the QOL score was significantly lower in the Fumaria group (p<0.001) at 8th week. About adverse events, the incidence of somnolence in the Fumaria group was significantly lower than in the cetirizine group (p<0.001). CONCLUSIONS: Fumaria vaillantii demonstrated its effects on CU later than cetirizine, but led to more permanent effects, better quality of life, and lower incidence of adverse events as compared to cetirizine. More clinical trials with higher populations are needed to achieve more conclusive results.
Background
Allergic diseases are a major problem for health systems because of the lack of accurate diagnosis and complete treatment for them. Urticaria is one of the major forms of these diseases that specifically affect the quality of life (QOL) [1]. Clinical features of urticaria are transient skin rash with itching and redness that can create swelling for 24 to 72 hours in the dermis and hypodermis [2]. More than 20% of the world’s population is affected by acute urticaria and more than 1% suffers from chronic urticaria (CU) during their lifetime. The incidence of acute urticaria is higher among young people, while CU mostly occurs in middle-aged women [3][4]. 40% of CU occurs with angioedema. Despite various proposed mechanisms, the exact mechanisms involved in the pathogenesis of urticaria are still unknown. The activation of mast cells causing histamine release, the infiltration of CD4+, T lymphocytes, monocytes, neutrophils, eosinophils, and basophils are the already well-known pathogenesis of this disease. Based on cutaneous late- phase reactions in CU, both T-helper 1 and T-helper 2 cells, it seems to be activated with the production of IFN- γ by the former cells and IL-4 and IL-5 by the latter [5]-[7]. However, due unsatisfactory response to anti-histamines in all patients, researchers are studying other factors involved in the pathogenesis of urticaria including the dysfunction of neuroimmunendocrine and autoimmunity [8]-[10].
The first suggestion for the treatment of urticaria is non-hypnotic antihistamines of which cetirizine are the most commonly used. Other interventions have been used as well, including corticosteroids, immunosuppressives, anti-leukotrienes, anti-receptor IgE antibodies, interferons, plasmapheresis, phototherapy, and intravenous immunoglobulins [9][10].
However, because of their unsatisfactory efficacy and side effects, investigations for new treatments seem necessary. Persian medicinal plants are valuable sources as new treatments for various disorders [11][12]. Fumaria vaillantii Loisel commonly known as earth smoke is a perennial medicinal plant from the Papaveraceae family. Its major chemical constituents 3 are benzylisoquinoline alkaloids, flavonoids and organic acids like fumaric acid [13] .
Various pharmacological activities have been attributed to the Fumaria species including anti- inflammatory, anti-histaminic, uremic pruritus treatment, anticancer [14][15], modulation of gastric acidity, antibacterial, antioxidant [16], treatment of the liver, gastrointestinal diseases and eczema [17]. The efficacy of Fumaria has been emphasized for the management of skin diseases, especially urticaria, in Persian medicine (PM) [18]. Thus, the aim of this single-blind, randomized, controlled trial is to compare the efficacy and tolerability of a dosage form made from Fumaria vaillantii with cetirizine in CU treatment.
Methods
Patients
10-70 years old male and female patients suffering from CU were recruited for the study. Patients were excluded from the study if they had undesirable conditions including uncontrolled cardiovascular, respiratory, hematological, urinary, immune system and connective tissue diseases, history of severe angioedema, seizure and intraocular pressure [19], sensitivity to cetirizine or Fumaria, or had received topical or oral corticosteroids in the previous seven days or antihistamines in the previous five days before randomization. Similarly, patients were excluded in case of annoying adverse events, unwillingness to continue to participate, or failure to follow-up regularly. Pregnant women and nursing mothers were also excluded from the trial.
Study design and therapeutic intervention
This study was a single center, single-blind, randomized, parallel group, phase III clinical trial of primary prospective-interventional studies with quantitative data, which was performed on human samples This trial was approved by the Ethics Committee of Tehran University of Medical Sciences (N#IR.TUMS.REC.1394.1292) and was registered at the Iranian Registry of Clinical Trials (N#IRCT2015112825275N1). CU patients who were referred to the Specialized Skin Clinic of Persian Medicine, faculty of Tehran University of Medical Sciences (TUMS), from March 5, 2016 to March 5, 2017 were recruited for the study after meeting the inclusion criteria. They were randomly included in the study after full familiarity with the study and obtaining a written consent form from them. Then, a patient’s basic data according to the guideline for CU [20], including the patient’s name, address, telephone number, gender, age, duration of disease, job, history of previous allergic diseases (asthma, allergic rhinitis, atopic dermatitis, and psoriasis), home and occupational exposures, family history of atopy, the patient’s vital signs, severity of the condition and quality of life were recorded in a questionnaire set (day 0). The patients were divided by blocked randomization method into either one of two treatment groups: Fumaria or cetirizine. The randomization process was done by a third person, who was not involved with the patients. The syrups were poured in containers with the same shape, color and with identical packaging. The intervention was carried out by the researcher, while the researcher were blinded to the group and medication.
The Fumaria vaillantii syrup (prepared by Persian Medicine Pharmaceutical Department professors of Tehran University of Medical Sciences) and the cetirizine syrup were prescribed twice daily in the in fasting (6 A.M and 6 P.M), each time as 10 cc, for four weeks. The patients were recommended to avoid spicy and canned food containing preservatives, which had effects in the development of urticaria [21]. The patients were visited at the end of the first and the fourth week of the trial and by phone at the end of the third week. A fallow-up visit was scheduled one month after the end of the project.
Formulation and standardization of Fumaria syrup
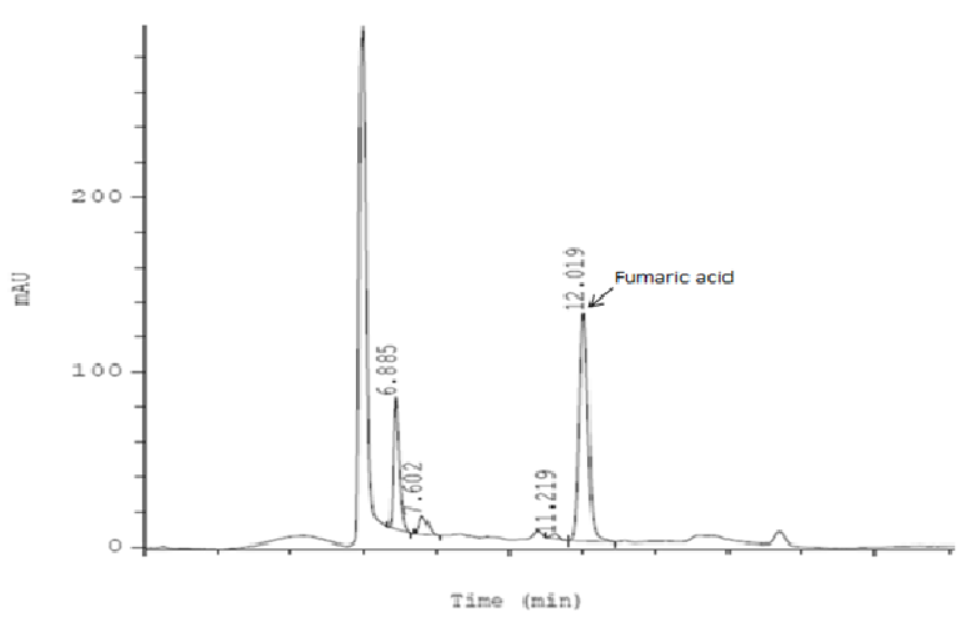
In this section, we used the Medicinal Plants Consort checklist. The dried aerial parts of Fumaria vaillantii Loisel. (Voucher No. PMP-335, herbarium of the faculty of pharmacy, Tehran University of Medical Sciences) was weighted accurately (25 g), grinded and macerated in distilled water (150 ml) for 24 hours at room temperature. The herbal extract was filtered (20 mesh) and was heated gently (40◦C) to become more concentrated. Separately, a sufficient amount of distilled water (350 ml) was heated to 85◦C. Then, sugar (200 g) was added. In order to achieve a thick syrup with suitable viscosity, the process of heating was continued. Then, the warm thickened syrup was filtered (20 mesh) immediately. As soon as the temperature of the syrup reached 40◦C, the concentrated herbal extract was added to the syrup. The whole mixture was gently heated (40◦C) until the syrup volume reached to 240 ml. Then, the heating was stopped. After complete cooling, the syrup was transferred to a dark pet (Type-3 bottle container). After obtaining the standard equation of fumaric acid and analyzing the syrup sample, the syrup was standardized according to its fumaric acid content by using cation-exchange high-performance liquid chromatography with UV detection (at 210 nm). A mobile phase of sulfuric acid 0.003 N was used. The performance of the HPLC system was isocratic. In order to obtain the standard calibration curve of fumaric acid, five diluted concentrations of standard fumaric acid were prepared and injected into the HPLC apparatus. In order to prepare a sample of Fumaria syrup for injection into the HPLC system, 1 ml of the syrup was placed in a 5 ml volumetric flask and diluted with distilled water. After the complete dissolution of the syrup, the solution was filtered with a special syringe filter before sample injection.The analysis of each sample was repeated thrice. The identification of the peak of fumaric acid related to the syrup sample was carried out by comparing its retention time with the standard ones Figure 1 . By placing the surface area of fumaric acid in the standard calibration equation of fumaric acid, the concentration of fumaric acid in the sample solution was calculated. Finally, the fumaric acid content in 5 ml of Fumaria syrup was calculated to be 0.12 mg.
Efficacy assessment
The primary endpoints to measure the effectiveness were the change from baseline Urticaria Activity Score (UAS) and the change in baseline Chronic Urticaria Quality of Life score (CU- Q2oL). UAS was defined as the sum of the hive number (without urticaria = 0, less than 20 = 1, 21-50 = 2, more than 50 = 3) and the itch score (no itching = 0, itching but not annoyingly = 1, itching, annoying but not impeding daily activities and sleep = 2, itching, painful and interfering with daily activities and sleep = 3). The total score of UAS would equal to 0-6. The total scores of UAS during each week turn to a score between 0 and 42 that was called a weekly benchmark or UAS-7, which was validated in a recent study to track CU severity [22]. A larger value suggested more severity of symptoms. A daily UAS form was completed by the patients every evening. The change of UAS-7 was calculated at the end of weeks 1, 4 and 8.
In addition, the standardized Persian version of QOL in CU questionnaire was used to assess the patients’ QOL at the end of weeks 4 and 8. Its validity and reliability, with the original in Italian, was approved and published under the supervision of Doctor Nima Rezaei [23]. This questionnaire had 23 questions categorized into three groups, which assessed the effectiveness of CU during 15 recent days on the patients’ QOL. The groups included disease symptoms (four questions), the patients’ activities (six questions), and social aspects (10 questions). The patients needed to select an option of not at all, low, moderately, high, and very high (between 0 and 4) for each question. The sum of the resulting scores came to a number between 0 and 92. A higher value was the sign of more negative impact of the disease on the QOL. The baseline scores of the UAS and QOL were assessed by the investigator on day 0.
Safety assessment
Safety in this study was assessed by evaluated vital signs (blood pressure, body temperature, heart rate, and respiratory rate) and adverse events according to the Common Terminology Criteria for Adverse Events Questionnaire (CTCAE). The possible adverse events of medications were evaluated and the researcher investigated the number, the severity, and the relationship of these adverse events with prescribed drugs.
Statistical analysis
A sample size of 37 patients per group was calculated to provide a statistical power of 80% at a two-sided level of significance of 5%, considering a minimum acceptable treatment difference of 4 in the score of quality-of-life and a standard deviation of 6, based on a previous study [24]. Assuming an approximately 5% attrition of patients from the study, the final sample size was calculated to be 39 patients in each arm.
The incidence of adverse events was compared between the groups by Fisher’s exact test. The continuous variables and the categorical variables were compared between the groups by two independent samples of t-test and Chi square. The changes of UAS and the scores of QOL in each group were analyzed by using an ANOVA model. Paired samples t-test was used to compare the continuous variables before and after interventions in individual groups Efficacy analyses were performed to compare the two groups by an Analysis of Covariance (ANCOVA) model.
All the statistical analyses were performed with Statistical Package for Social Sciences (SPSS) version 18. All the reported p-values were two-sided and p <0.05 was considered to be statistically significant. Intention to treat (ITT) was applied in the analysis.
Results
Study Population
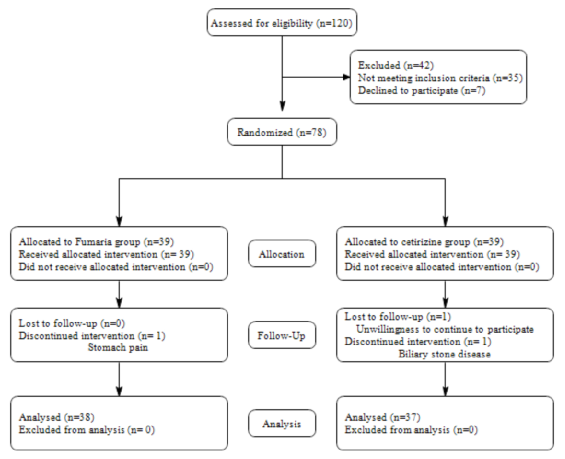
March 5, 2016 to March 5, 2017, 120 CU patients referred to Skin Clinic of Persian Medicine of TUMS were assessed and 78 patients were randomized and allocated to one of the two treatment groups. There were 39 patients in the Fumaria group and 39 patients in the cetirizine group. 42 patients were excluded from the study for not meeting the inclusion criteria (n=35) and for declining to participate (n=7). Three patients were lost during follow-up, including one in the Fumaria group due to an incidence of stomach pain and two in the cetirizine group, with one for biliary stone disease and one for unwillingness to continue to participate. Finally, 75 patients were analyzed and completed the study. A flow diagram of the clinical study is shown in Figure 2 .
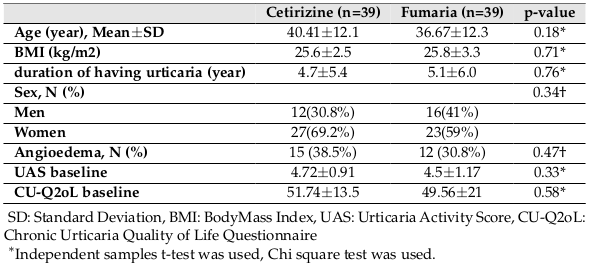
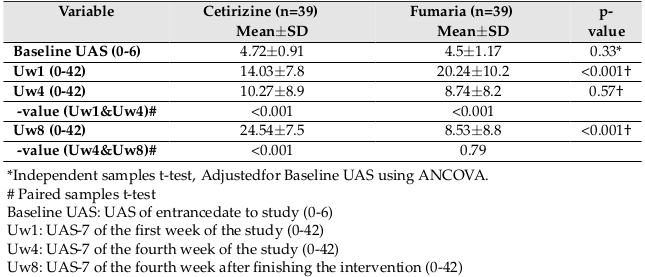
The baseline characteristics of the two groups are summarized in Table 1 . Accordingly, no significant difference in age, sex, body mass index (BMI), duration of having urticaria and angioedema, and the baseline UAS and QOL between the two groups was reported.
UAS
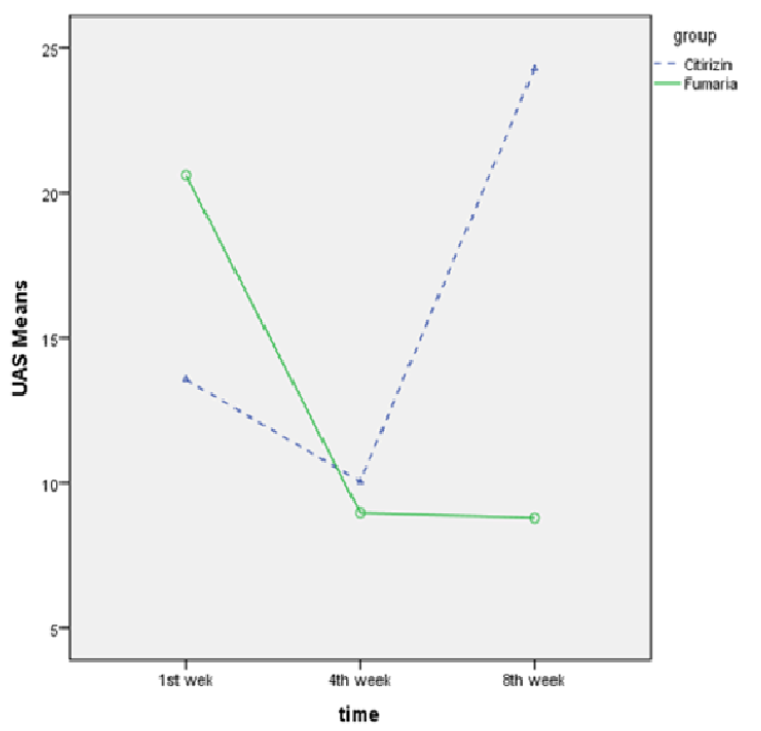
The results showed that the UAS was significantly higher in the Fumaria group than in the cetirizine group after the first week of follow-up (p<0.001), but significant difference was not seen between the two groups at week 4 (p=0.57). At 8th week (one month after finishing the intervention), the UAS score of the cetirizine group was significantly higher than that of the Fumaria group (p<0.001).
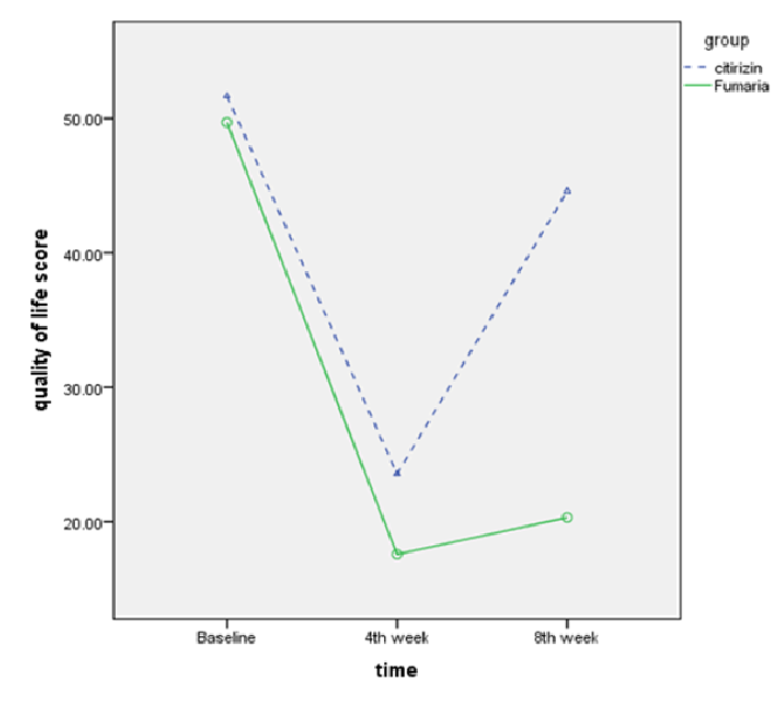
Also the mean UAS-7 score at 4th week versus the first week decreased from 14.03±7.8 to 10.27±8.9 (p<0.001) in cetirizine group and decreased from 20.24±10.2 to 8.74±8.2 (p<0.001) in Fumaria group. Also, the mean UAS-7 score at 8th week versus 4th week, increased from 10.27±8.9 to 24.54±7.5 (p<0.001) in cetirizine group and decreased from 8.74±8.2 to 8.53±8.8 (p=0.79) in Fumaria group Figure 3 and Table 2 .
Quality of life
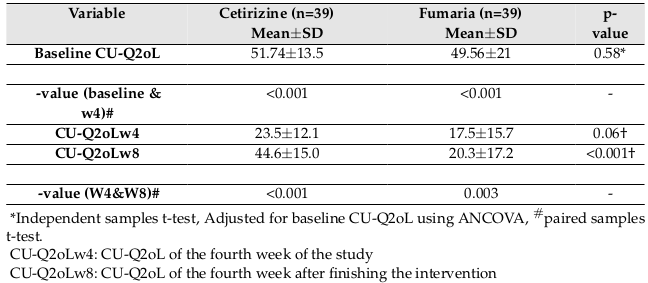
The results showed that after finishing the intervention, there was a lower CU-Q2oL in the Fumaria group, but this difference was not statistically significant (p=0.06). Four weeks after finishing the intervention, the QOL score was significantly lower in the Fumaria group (p<0.001). The mean QOL score at the end of 4th week decreased from 51.74±13.5 to 23.5±12.1 (p<0.001)
in cetirizine group and decreased from 49.56±21 to 17.5±15.7 (p<0.001) in Fumaria group. Also the mean QOL score at the end of 8th week versus 4th week, increased from 23.5±12.1 to 44.6±15.0 (p<0.001) in cetirizine group and increased from 17.5±15.7 to 20.3±17.2 (p=0.003) in Fumaria group Figure 4 and Table 3 .
Adverse events
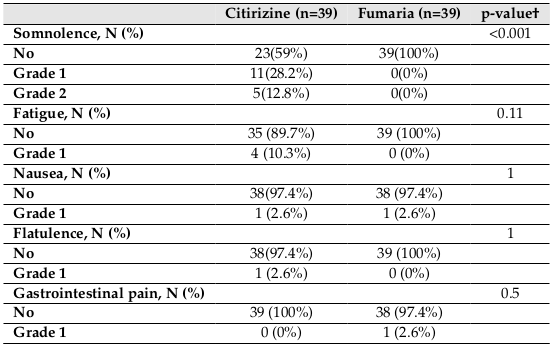
The incidences of adverse events in the two groups are shown in Table 4 . In the cetirizine group, the most common adverse events were somnolence and fatigue, respectively. 16 subjects (41%) in the cetirizine group complained of somnolence (grade 1: 11 patients, and grade 2: 5 patients), and no patient in the Fumaria group complained of somnolence. Accordingly, there was a significantly lower incidence of somnolence in the Fumaria group than in the Cetirizine group(p<0.001). The incidences of other adverse events between the two groups did not demonstrate significant difference. In the Fumaria group, one patient reported nausea and another complained of stomach pain.
Vital signs
During the study, none of the patients reported of any disruption in the vital signs or any life- threatening adverse event.
Discussion
Urticaria is a common skin disease whose cause is idiopathic in three-quarters of the patients [25] and none of the available conventional medicines can successfully manage the disease [8][26]. In a case series (2013), 104 case reports of allergic reactions to a variety of antihistamines were listed, with the highest sensitivity rate assigned to cetirizine and the most allergic reaction was urticaria with or without angioedema [27]. A study by Guevara et al. reported various side effects for cetirizine, including somnolence (over 60%), fatigue (over 40%), nausea, headache, impaired vision, dizziness, diarrhea, constipation and abdominal pain [24]. Based on these problems, the use of complementary and alternative medicines such as Persian medicine is recommended. In Persian medicine, many topical and oral medications have been suggested for the treatment of urticaria (Sherry in PM) [28][29].
In this clinical trial, the effect of Fumaria syrup, a Persian medicine remedy, was compared with cetirizine syrup on CU. Recommended daily dose of fumaria as infusion is 2-4 g [30]. The daily intake of fumaria by syrup in this study was about 2 g per day. The daily intake of cetirizine was 20 mg per day in this study. Recommended daily dose for cetirizine is between 10 to 40 mg depending on severity of disease symptoms [31].
Based on the initial results from this study, both Fumaria and cetirizine syrups reduced the symptoms of CU. The onset of cetirizine’s effect was earlier than that of Fumaria and caused a significant change in the symptoms of patients in the first few days, while the therapeutic effects of Fumaria appeared over time in the patients. At the end of the fourth week, almost the same effects could be seen from both syrups. A month after drug withdrawal, the symptoms in the cetirizine group were similar to the time before receiving the drug, but in the Fumaria group, the symptoms still showed a significant decrease in many patients. The delayed and constant effects of medicinal plants on CU have also been confirmed by other studies [32]. Also, in terms of improved QOL, both syrups made a significant improvement in the QOL of patients. However, one month after stopping of medication, the QOL of patients in the Fumaria group was significantly better than in the cetirizine group. Therefore, compared to cetirizine syrup, Fumaria syrup led to reduced symptoms of hives, especially in the long term and further improved the QOL of the patients. In this study, there were no life-threatening side effects and the incidences of adverse events were higher in the cetirizine group. 16 people complained of somnolence in the cetirizine group. The severity grade was 2 in five patients, which was significantly higher than that in the Fumaria group.
The anti-inflammatory and anti-histaminic effects of Fumaria were confirmed in an animal model. Based on experiments performed in 2007, the oral intake of alcoholic extract of Fumaria after carrageenan and histamine induced hind paw edema, clearly and dose-dependently, was effective in improving acute inflammations in exudative and proliferative phases and chronic inflammations [33].
One of the major chemical components of Fumaria is fumaric acid. Dimethyl fumarate (DMF) is a potent anti-inflammatory medication for psoriasis and it has also been shown to suppress inflammation in other chronic inflammatory diseases. DMF is metabolized to fumaric acid, which enters the citric acid cycle and thereby inhibits inflammatory processes. DMF reduces the pro- inflammatory contribution of several cell types including T lymphocytes, mononuclear blood cells, dendritic cells, endothelial cells, and keratinocytes [34]. Some studies have shown that monomethyl fumarate can increase IL-4, an anti-inflammatory interleukin, or can decrease IFN- γ, an inflammatory factor [15]. Also, caffeic acid (a phenolic component), narceimine, narlumidine, adlumidine, and protopine nitrate in Fumaria vaillantii exhibit anti-inflammatory activities [35][36].
The accepted effects of Fumaria on liver dysfunction (the main cause of CU in PM) confirm that it can be helpful in CU treatment [29][37][38]. To this, Shamsi et al. (2005) evaluated the effects of Pitkirya capsules, a combination of six plants including Fumaria, with placebo on 108 patients with CU in a triple-blind clinical trial. In that study, all the disease symptoms showed significant reduction in the drug group as compared to the placebo group. In addition, other than 14 cases of mild drowsiness, no other side effects were reported, without any change in the laboratory tests [39].
According to the results of this study, it seems that Fumaria, an herb with no harmful adverse events in permissible therapeutic doses, with anti-inflammatory activity would improve CU symptoms.
Conclusion
Fumaria vaillantii, a Persian medicine remedy, initially demonstrated its effects on CU later than cetirizine, but led to more permanent effects, better quality of life, and lower incidences of adverse events as compared to cetirizine. More clinical trials with higher populations are needed to achieve more conclusive results.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
Chronic urticarial = CU, Common Terminology Criteria for Adverse Events Questionnaire = CTCAE, Quality of Life Questionnaire = CU-Q2oL, Tehran University of Medical Sciences = TUMS, Urticaria Activity Score = UAS
Ethics approval and consent to participate
This trial was approved by the Ethics Committee of Tehran University of Medical Sciences (N# IR.TUMS.REC.1394.1292)
Competing interests
Authors have no conflict of interest.
Funding
This study has been partially supported by Tehran University of Medical Sciences; Grant No 94 03 86 30114.
Authors’ contributions
Fatemeh Eghbalian: Literature search, Design, Clinical studies, Data acquisition, Manuscript preparation. Nafiseh Esmaili & Mehrdad Karimi: Concepts, Design, Definition of intellectual content, Clinical studies, Manuscript editing. Roja Rahimi & Fahimeh Mohajerani: Formulation and production of drug, Concepts, Manuscript editing. Akramosadat Atyabi: Clinical studies, Concepts, Manuscript review. Hamid Reza Tohidinik: Concepts, Data analysis, Manuscript review. Laila shirbeigi: Concepts, Design, Clinical studies, Manuscript editing, Guarantor. All authors read and approved the final manuscript.
References
-
GN
Dakhale,
AT
Shinde,
MS
Mahatme,
SK
Hiware,
DB
Mishra,
JI
Mukhi,
AM
Salve.
Clinical effectiveness and safety of cetirizine versus rupatadine in chronic spontaneous urticaria: a randomized, double-blind, 6-week trial. International journal of dermatology.
2014;
53
:
643-649
.
View Article PubMed Google Scholar -
S
BEfA.
Harrison s principle of internal medicine. New York: MC Graw Hill.
2008;
:
1846-1865
.
-
SS
Saini.
Chronic spontaneous urticaria: etiology and pathogenesis. Immunology and Allergy Clinics.
2014;
34
:
33-52
.
View Article Google Scholar -
S
Yadav,
A
Upadhyay,
al
Bajaj AK et.
Chronic urticaria: An overview. Indian Journal of Dermatology.
2006;
51
:
171
.
View Article Google Scholar -
S
Jain.
Pathogenesis of chronic urticaria: an overview. Dermatology research and practice 2014.
2014a
.
-
S
Jain.
Pathogenesis of chronic urticaria: an overview. Dermatology research and practice 2014.
2014b
.
-
AP
Kaplan,
M
Greaves.
Pathogenesis of chronic urticaria. Clinical & Experimental Allergy.
2009;
39
:
777-787
.
View Article PubMed Google Scholar -
LP
Viegas,
MB
Ferreira,
AP
Kaplan.
The maddening itch: an approach to chronic urticaria. J Investig Allergol Clin Immunol.
2014;
24
:
1-5
.
PubMed Google Scholar -
ER
Kavosh,
DA
Khan.
Second-Generation H 1-Antihistamines in Chronic Urticaria. American journal of clinical dermatology.
2011;
12
:
361-376
.
PubMed Google Scholar -
al
Rathi SK et.
Comparison of levocetirizine and cetirizine in chronic idiopathic urticaria. Indian Journal of Dermatology.
2004;
49
:
130
.
-
M
Mehriardestani,
A
Aliahmadi,
T
Toliat,
R
Rahimi.
Medicinal plants and their isolated compounds showing anti-Trichomonas vaginalis-activity. Biomedicine & Pharmacotherapy.
2017;
88
:
885-893
.
View Article PubMed Google Scholar -
M
Salehi,
H
Karegar-Borzi,
M
Karimi,
R
Rahimi.
Medicinal plants for management of gastroesophageal reflux disease: A review of animal and human studies. The Journal of Alternative and Complementary Medicine.
2017;
23
:
82-95
.
View Article PubMed Google Scholar -
A
Meyer,
P
Imming.
Benzylisoquinoline alkaloids from the Papaveraceae: the heritage of Johannes Gadamer (1867-1928). Journal of natural products.
2011;
74
:
2482-2487
.
View Article PubMed Google Scholar -
FHA
Tabrizi,
S
Irian,
A
Amanzadeh,
F
Heidarnejad,
H
Gudarzi,
M
Salimi.
Anti- proliferative activity of Fumaria vaillantii extracts on different cancer cell lines. Research in pharmaceutical sciences.
2016;
11(2)
:
152-159
.
PubMed PMC Google Scholar -
JAGW
Blajer,
K
Mariusz.
The Inverse Simulation Study of Aircraft Flight Path Reconstruction. Transport.
2002;
XVII
:
103-107
.
-
U
Mandal,
KM
Ali,
K
Chatterjee,
D
De,
A
Biswas,
D
Ghosh.
Management of experimental hypochlorhydria with iron deficiency by the composite extract of Fumaria vaillantii L. and Benincasa hispida T. in rat. Journal of natural science, biology, and medicine.
2014;
5
:
397
.
-
L
Rakotondramasy-Rabesiaka,
JL
Havet,
C
Porte,
H
Fauduet.
Solid-liquid extraction of protopine from Fumaria officinalis L.-analysis determination, kinetic reaction and model building. Separation and Purification Technology.
2007;
54
:
253-261
.
-
SMHA
Shirazi.
Makhzan-ol-Advieh [Storehouse of medicaments]. 1992
.
-
L
Skidmore-Roth.
Mosby’s Handbook of Herbs and Natural Supplements. Mosby.
2006
.
-
T
Zuberbier,
W
Aberer,
R
Asero,
C
Bindslev-Jensen,
Z
Brzoza,
GW
Canonica,
MK
Church,
LF
Ensina,
A
Giménez-Arnau,
K
Godse.
The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy.
2014;
69
:
868-887
.
View Article Google Scholar -
G
Akoglu,
N
Atakan,
B
Cakir,
O
Kalayci,
M
Hayran.
Effects of low pseudoallergen diet on urticarial activity and leukotriene levels in chronic urticaria. Archives of dermatological research.
2012;
304
:
257-262
.
View Article PubMed Google Scholar -
I
Jáuregui,
FJO
de Frutos,
M
Ferrer,
A
Giménez-Arnau,
J
Sastre,
J
Bartra,
M
Labrador,
JF
Silvestre,
A
Valero.
Assessment of severity and quality of life in chronic urticaria. J Investig Allergol Clin Immunol.
2014;
24
:
80-86
.
PubMed Google Scholar -
M
Movahedi,
M
Tavakol,
P
Mohammadinejad,
I
Baiardini,
F
Braido,
M
Gharagozlou,
A
Aghamohammadi,
M
Nabavi,
A
Dabbaghzade,
al
Tavakol Z et.
The persian version of the chronic urticaria quality of life questionnaire: factor analysis, validation, and initial clinical findings. Iranian Journal of.
2014;
Allergy
:
Asthma and Immunology 13(4)
.
-
E
Guevara-Gutierrez,
S
Bonilla-Lopez,
S
Hernández-Arana,
A
Tlacuilo-Parra.
Safety and efficacy of cetirizine versus cetirizine plus ranitidine in chronic urticaria: Double-blind randomized placebo-controlled study. Journal of Dermatological Treatment.
2015;
26
:
548-550
.
View Article PubMed Google Scholar -
H
Tanizaki,
Y
Yamamoto,
S
Nakamizo,
A
Otsuka,
Y
Miyachi,
K
Kabashima.
Comparison of the efficacy of olopatadine and fexofenadine in chronic idiopathic urticaria patients: a crossover study. Pharmacology.
2015;
95
:
32-35
.
View Article Google Scholar -
JH
Kim,
SS
Park.
Retrospective case series on Gwakhyangjeonggi-san prescribed to patients with chronic urticaria. Complementary therapies in medicine.
2015;
23
:
806-809
.
View Article PubMed Google Scholar -
AA
Shakouri,
SL
Bahna.
Hypersensitivity to antihistamines. In Allergy and asthma proceedings.
2013;
34
:
488-496
.
-
Khorasani
Aghili,
MH.
Shirazi,
Hekmah
kholasatol.
Qom:, Ismailis. 1385 . AD. N. C 3. P. 65.
.
-
Jahan Mohammad Azam
Nazem,
Eksor-e-Azam.
Teharan: Iran University of Medical Sciences, Institute of Medical History, Islamic and Complementary Medicine; 2008. 2008
.
-
Committee on Herbal Medicinal Products (HMPC).
Community herbal monograph on Fumaria officinalis L., herba, 2011 EMA/HMPC/574766/2010.
.
-
JA
Bernstein,
DM
Lang,
DA
Khan,
T
Craig,
D
Dreyfus,
F
Hsieh,
J
Sheikh,
D
Weldon,
B
Zuraw,
al
Bernstein DI et.
The diagnosis and management of acute and chronic urticaria: 2014 update. Journal of Allergy and Clinical Immunology.
2014;
133
:
1270-1277
.
View Article PubMed Google Scholar -
M
Yan,
F
Ye,
Y
Zhang,
X
Cai,
Y
Fu,
X
Yang.
Optimization model research on efficacy in treatment of chronic urticaria by Chinese and Western Medicine based on a genetic algorithm. Journal of Traditional Chinese Medicine.
2013;
33
:
60-64
.
View Article Google Scholar -
C
Rao,
A
Verma,
P
Gupta,
M
Vijayakumar.
Anti-inflammatory and anti-nociceptive activities of Fumaria indica whole plant extract in experimental animals. Acta Pharmaceutica.
2007;
57
:
491-498
.
View Article PubMed Google Scholar -
P
Seidel,
M
Roth.
Anti-inflammatory dimethylfumarate: a potential new therapy for asthma?. Mediators of inflammation 2013.
2013
.
-
S
Srivastava,
GP
Choudhary.
Pharmacognostic and pharmacological study of Fumaria vaillantii Loisel: a review. Journal of Pharmacognosy and Phytochemistry.
2014;
3(1)
:
194-197
.
-
CP
Khare.
Encyclopedia of Indian medicinal plants. NewYork: Springes-Verlag Berlin Heidelberg.
2004
.
-
G
Jelodar,
S
Nazifi.
Effect of fumitory, coriander seed and madder on serum biochemical parameters of diabetic rats. Pathophysiology.
1998;
1001
:
175
.
View Article Google Scholar -
SK
Maurya,
A
Seth.
Potential medicinal plants and traditional Ayurvedic approach towards urticaria: an allergic skin disorder. International Journal of Pharmacy and Pharmaceutical Sciences.
2014;
6
:
172-177
.
-
Y
Shamsi,
H
Kumar,
SA
Tamanna,
EA
Khan.
Effect of a polyherbal Unani formulation on chronic urticaria. Indian Journal of Traditional Knowledge.
2006;
5(2)
:
279-283
.
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 6 (2018)
Page No.: 2389-2401
Published on: 2018-06-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4964 times
- Download PDF downloaded - 1382 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress