Abstract
Introduction: This study aims to elucidate the possible ameliorative effect of Cymbopogon citratus (CC), i.e. lemon grass, in a Doxorubicin (Dox)-induced male testicular damage and infertility model. Moreover, the anticancer role of the main active constituents of CC (Apigenin and Quercetin) was evaluated on prostatic and colon cancer (PC-3 and HCT-116 carcinoma cell lines, respectively).
Methods: In vitro studies of the cell lines were carried out to determine the IC50 of each active constituent of CC, in order to select the most suitable dose for in vivo studies. For the in vivo studies, 24 rats were divided into 4 groups: Group 1 received saline treatment (negative control), Group 2 received treatment with CC (300 mg/kg body weight (b.w.)), Group 3 received Dox treatment (25 mg/kg b.w. intraperitoneally (i.p.)) on day 9 of the study, and Group 4 received CC treatment followed by Dox treatment on day 9 of the study. All the treatments were administered orally for 10 consecutive days.
Results: The data revealed that Apigenin and Quercetin (AQ) had greater potency against PC-3 cells than HCT-116 cells. In vivo studies demonstrated that Dox treatment induced testicular damage and increased nitrosative stress, inflammation, and cell death, while decreasing cell energy markers for testes. In contrast, rats treated with CC only, or in parallel with Dox, showed ameliorative responses against Dox; they also showed greater testicular activity than normal, which reflected the ability of CC to significantly improve reproductive health performance.
Conclusion: In conclusion, CC inhibits the activity and growth of PC-3 and HCT-116 carcinoma cell lines and, moreover, the whole extract of CC ameliorates testicular dysfunction induced by Dox.
Background
Anticancer drugs, such as Cyclosporine, Allopurinol, Colchicine, Sulfasalazine, Spironolactone, and other drugs (such as marijuana and alcohol), especially if used in large quantities, may slow down and affect sperm production 1. About two-thirds of male patients with cancer who are treated with anticancer drugs have trouble forming sperm in the testicle, leading to sperm deficiencies and/or loss of function 2. Chemotherapy is the treatment to eliminate cancer cells. Drugs have been used in the treatment of many diseases; in fact, there are currently more than twenty drug species in use. The chemotherapeutic drug is distributed through the blood throughout the body, which gives a greater chance of eliminating cancerous cells in the body that may have migrated from the primary tumor to another organ in the body 3. As aforementioned, chemotherapy for cancer may disrupt testicular function, either permanently or temporarily, after a period of administration. It can destroy growing cancerous cells which growing more rapidly than normal cells 4. As the cancer cells grow, they multiply and divide at a rate greater than that of the rest of the body's normal cells, yet chemotherapy is able to destroy them 5. Some natural cells in the body grow and multiply quickly too. Thus, chemotherapy cannot differentiate between the normal cells and cancerous cells. Thus, the side effect of chemotherapy, i.e. non-specific killing of normal cells, can be a huge downturn. However, after chemotherapy is ended, the effects on non-cancerous cells will return to normal 6. Doxorubicin (Dox) is an anticancer drug that is widely used to treat various types of cancers, and can cause side effects in the short term. Following administration into the vein, Doxorubicin penetrates the cells and forms stable complexes with DNA molecules, thereby killing the cancer cells preferentially 7. In order to multiply, cancer cells need to constantly replicate samples of DNA, which are no longer able to do so when complexed with cisplatin. However, this therapy has major obstacles. Within an hour or even half an hour after injection, some patients have side effects or develop many disorders 8. Cymbopogon citratus (CC), i.e. lemon grass, is a long-standing perennial herb grown in the tropical regions of Asia and India 9. It has a number of health and medicinal benefits which include anticancer, antioxidant, antibacterial, antifungal, and antimicrobial properties 10. The benefits of lemon grass are that it can relieve gastrointestinal and stomach disorders, treat insomnia and fever, contribute to the treatment of rheumatoid arthritis, help maintain blood cholesterol levels, promote the health of the immune system, help in the treatment of obesity, type II diabetes and cancer, and contribute to the removal of toxins from the body 1112. The PC-3 cell line is one of the human prostate cancer cell lines used in research. These cells are helpful in examining the biochemical alterations in prostatic cancer and in surveying their reaction to chemotherapeutic agents 13. The HCT-116 cell line is a human colon cancer cell line that has been used to investigate or induce a rat colon cancer model14. The aim of the present study was to investigate the possible ameliorative effects of Dox in combination with CC on the reproductive male function. In addition, the study aimed to evaluate the potential anticancer activity of the main active constituents of CC (Apigenin and Quercetin) on growth inhibition of PC-3 and HCT-116 cell lines.
Methods
Plant extracts
Plant materials were identified by the Applied Research Center for Medicinal Plants (ARCMP) in Egypt. The materials were dried in a lyophilizer at -50ºC until dried, then grinded. The ethanolic extract was prepared by macerating the CC leaves with ethanol (70%) overnight. The process was repeated three times. Then, the extraction was filtered and evaporated to dryness in a vacuum by lyophilization/freeze-drying. The filtrate (ethanolic extract) was then stored at -20◦C until further use 15.
Animals
Twenty-four male Wister rats (120-150 g) from the animal facility of the National Organization for Drug Control and Research (NODCAR) in Egypt were used in the study. The animals were kept at room temperature (22±1◦C), with 12 h light and free access to diet and tap water.
Measurement of potential cytotoxicity of Apigenin and Quercetin on PC-3 and HCT-116 cell lines by Sulfo-Rhodamine-B (SRB) staining assay
The potential cytotoxic effects of the compounds were tested using the method of Skehan and Storeng 16, which was as follows:
Determination of the active compounds of Cymbopogon Citratus (CC) by high performance liquid chromatography (HPLC)
Flavonoids of CC leaves were extracted according to the method outlined by Fernandez de Semon et al. 17 and Dimitrios et al. 18.
Experimental design
Rats were divided into four groups (n=6 rats per group), as follows:
-Group 1: rats received saline orally every day for 10 days (normal control group).
-Group 2: rats received the ethanolic extract of CC every day for 10 days (300 mg/kg b.w., per os (p.o.)) 19.
-Group 3: rats received Dox (25 mg/kg b.w., i.p.) 24 h before decapitation (positive control group) 20.
-Group 4: rats received CC orally every day for 10 days and then were injected with Dox for 24 h before decapitation.
The testis tissues were kept at -80◦C until use for measurement of nitrosative stress, arachidonic acid (AA), malondialdehyde (MDA), glutathione (GSH), ATP, phosphatidylcholine, and phosphatidylserine, as well as genomic DNA analysis (on 1% agarose). A part of the testis tissue was fixed in 10% formaldehyde for histopathological examination.
Determination of tissue nitric oxide (NO) (µmol/g tissue)
Nitric oxide (NO) levels were determined with anion exchange column (Hamilton PRP-X100) using the parameters (150*4.1mm, 10 μm, mobile phase 45:55 of 0.1 M NaCl-methanol); wavelength was adjusted to 230 nm, according to HPLC procedure 21.
Determination of tissue MDA (nmol/g tissue) by HPLC
HPLC, sample preparation, and chromatographic conditions were carried out with some modifications of protocols from Algohary et al. 22.
Determination of tissue ATP (µmol/g tissue) by HPLC
HPLC, sample preparation, and chromatographic conditions were carried out with some modifications of protocols from Abd-Elrazek et al. 23.
Phospholipid extraction
Phospholipid extraction and determination were done according to previous protocols from study by Yassin et al. 24.
Determination of arachidonic acid (AA)
Arachidonic fatty acid was determined using gas chromatography. Standards were prepared in hexane:chloroform (1:1) and combined into a single fatty acid mixture. Extraction of the serum samples was prepared with Folch reagent, chloroform:methanol (2:1), then vortexed for 5 min and centrifuged at 4000 rpm for 10 min. Samples were evaporated at room temperature prior to derivatization. Finally, the supernatant was mixed with 2 ml of 95% methanol:sulfuric acid, then extracted with hexane for injection 25.
Extraction of genomic DNA
DNA extraction and detection of apoptosis (DNA fragmentation assay) were done according to the modifications from Hassab El-Nabi et al. 26.
Agarose gel electrophoresis for DNA fragmentation
Gel electrophoresis was used for separation and analysis of macromolecules and their fragments, based on size and charge. The 1% agarose gel was prepared according to the method of Awwad et al. 27.
Histological examination of testis sections
After the experimental period, animals were sacrificed and the testis section removed immediately, sliced, and washed in saline. The tissue pieces were preserved in 10% formalin for histopathological studies, then the pieces were processed and embedded in paraffin wax. Sections were taken and stained with hematoxylin and eosin (H&E) and photographed 28.
Statistical analysis
The data were demonstrated as mean±SEM (standard error of the mean). One-way ANOVA was carried out and statistical comparisons among groups were performed with Duncan’s test 29 using a statistical package of social science (SPSS) software (17.5). All analyses were two-tailed, and p<0.05 was considered as significant for all the statistical analyses in the study.
Results
Anti-proliferative activity or cytotoxicity of Apigenin and Quercetin on human prostate cancer (PC-3) and human colon cancer (HCT-116) cells
The SRB assay was used to assess the potential cytotoxic effects of Apigenin and Quercetin on human PC-3 prostate cancer cells and human HCT-116 colon cancer cells at different concentrations. The anti-proliferative activity on cancer cells was expressed as the IC50 value. IC50 is the inhibitory concentration that causes 50% inhibition of the cancer cell population. Table 1 and Figure 1 show that the cytotoxicity of Apigenin and Quercetin (AQ) on PC-3 and HCT-29 increases with increasing drug concentration. Thus, AQ had a dose-dependent effect on the cell viability, with cell lysis occurring after 48 h of drug treatment. The IC50 for A was 10.9 μg/ml and for Q was 11.6 μg/ml on PC-3 cells; the IC50 for A was 36.0 μg/ml and for Q was 31.8 μg/ml on HCT-116 cells.
| Cell Lines | Compounds | |
| Apigenin | Quercetin | |
| Prostate cancer (PC-3) | 10.9 μg/ml | 11.6 μg/ml |
| Colon cancer (HCT-29) | 36.0 μg/ml | 31.8 μg/ml |
Determination of the active compounds of CC leaves (Apigenin and Quercetin) by HPLC
The anticancer activity of flavonoids has been well-documented in the literature. Moreover, flavonoids have been reported for their anticancer activity. Figure 2 shows that CC leaves contain major components with a high amount of flavonoids: Quercetin (c), followed by Apigenin (b).
As shown in Table 2, Dox increases the reductive stress marker MDA, decreases the endogenous antioxidant marker GSH, increases the nitrosative stress marker NO, decreases the precursor of PGE-2 (AA), and increases testicular cell energy (ATP), in comparison with the control group. In contrast, CC treatment alone increases testicular function, and ameliorates the negative effects induced by Dox (when CC+Dox are used in combination).
| Groups | Parameters | ||||
| MDAnmol/g tissue | GSH μmol/g tissue | NOμmol/g tissue | AAμmol/g tissue | ATPμmol/g tissue | |
| Control | 35.02 ± 0.97 | 12.02 ± 0.32 | 0.394 ± 0.01 | 139.4 ± 3.7 | 28.02 ± 0.79 |
| CC | 26.94 ± 0.76a | 16.19 ± 0.37a | 0.302 ± 0.01a | 117.3 ± 3.12a | 22.76 ± 0.61a |
| Dox | 108.1 ± 2.80a | 7.031 ± 0.20a | 1.813 ± 0.05a | 66.02 ± 1.7a | 14.02 ± 0.38a |
| Dox+CC | 79.02 ± 1.90a | 9.701 ± 0.29ab | 1.424 ± 0.03ab | 85.07 ± 2.3ab | 19.03 ± 0.53ab |
As shown in Table 3, Dox decreases the reductive stress marker MDA, decreases endogenous antioxidant marker GSH, increases the nitrosative stress marker (NO), decreases the precursor of PGE-2 (AA), and increases testicular cell energy (ATP), in comparison with the control group. In contrast, CC alone increases testicular function and/or ameliorates the negative effects induced by Dox in the combination treatment group.
| Groups | Parameters | ||||
| Total lipid (TL)(mg/g testis) | Phospholipids (PL)(mg/g testis) | PC(μg/g testis) | (μg/g testis) | PC/PS | |
| Control | 14.02 ± 0.38 | 9.521 ± 0.25 | 398.2 ± 11.0 | 99.01 ± 2.71 | 4.052 ± 0.10 |
| CC | 16.70 ± 0.45 | 10.21 ± 0.26 | 431.6 ± 11.8a | 89.62± 2.32a | 4.244 ± 0.11 |
| Dox | 9.412 ± 0.25a | 6.643 ± 0.20 | 234.3 ± 6.61a | 135.5 ± 3.63a | 1.783 ± 0.05a |
| Dox+CC | 10.33 ± 0.28ab | 8.941 ± 0.25 | 281.1 ± 7.40ab | 111.2 ± 2.91ab | 2.561 ± 0.07ab |
Effects of CC ethanolic extracts alone or in combination with Dox-induced male reproductive toxicity, and effects on genomic DNA concentration in rat testis
Figure 3 demonstrates the identification of DNA of rat testis (by 1% agarose gel electrophoresis) after various treatments. The resulting bands were compared against the DNA Ladder (100-3000 bp). It was observed, from the DNA results, that DNA appeared to be damaged after treatment with Dox (which induces toxicity in rat tests) at 25 mg/kg b.w. i.p. However, pre-administration with CC in combination with Dox, or administration of CC alone, reduced the damage. All DNA bands were observed within the same molecular weight (more than 2000 bp).
Our results demonstrated that in the group with Dox-induced toxicity of rat testis, the DNA damage was clearly observed; this may be due to the oxidative stress and cell membrane damage as a result of the toxic effects of free radicals of lipid peroxidation, which act on DNA, membrane proteins, and lipids. It has been reported that the anti-proliferative effect of some medicinal plants, such as CC, is due to active flavonoid compounds which reduce DNA damage 30. Thus, our findings are in accordance with previous results. Administration of the CC ethanolic extract in rats produced a significant increase in DNA content in both induced and non-induced toxicity groups (less damage in the latter). This enhancement in DNA concentration following CC treatment was in a higher percentage in the non-induced toxicity group (than the induced group). The effect of CC on DNA appeared to be dose-dependent.
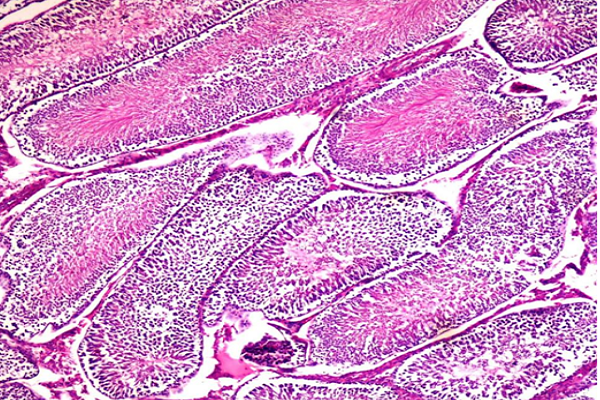
Histological examination of testis tissues (Figure 4a) showed normal cell structure and seminiferous tubules. Meanwhile, the Dox-treated group (Figure 4b) resulted in a reduction in spermatogenic lineage (T) and seminiferous tubules with vacuolation. Pre-treatment with CC alone (Figure 4c) or in combination with Dox (Figure 4d) for 10 days ameliorated the effect of Dox-induced toxicity on testis tissues. The data obtained in the study suggested that the CC extract is conceivably defensive against Dox-induced male reproductive damage. Biochemical investigations were affirmed by histopathological data which showed damage induced by Dox after treatment (Figure 4b).
Discussion
The testicles deliver the male gametes and male sexual hormones (androgens). Gonadal dysfunction is a common consequence of cytotoxic chemotherapy 31. Cymbopogon citratus (CC) is popularly known as lemongrass. CC has gained great interest because it is among the commercially valuable essential oils and also widely used in food technology as well as in traditional medicine 32. Nowadays, herbal remedies (such as CC) are a solution for patients with influenza, elephantiasis, pneumonia, and vascular issues. Data obtained from our study show that, indeed, CC has anticancer and cytoprotective effects against Dox-induced testicular dysfunction. Doxorubicin, an anthracycline antibiotic, is a generally utilized anticancer agent. Dox is integral to intercalation and DNA biosynthesis by inhibiting the progression of topoisomerase II, which decreases the mechanical action for cutting DNA strands and relaxing DNA tangles and supercoils 33. Dox suppresses the hydrolysis of ATP and topoisomerase Type-I, which change the linking number of circular DNA. Another stress that may be established after Dox induction is the generation of toxic reactive oxygen species (ROS) through disruption of transcription. First and foremost, the Dox-treated group showed a significant increase in MDA, NO, AA, and PS, and a decrease of GSH, ATP, TL, PL, and PC, in comparison with the control group. Compared to results of previous research studies, the results obtained in our study are consistent. Like other studies, our study shows the harmful effect of Dox and acceleration of lipid peroxidation, resulting in maximization of MDA levels and other inflammatory markers. According to pathogenesis features which occur through Dox-induced testicular toxicity, ROS has been implicated. Testicular lipid synthesis and cell membrane formation may be disrupted after Dox treatment, and PL may be degenerated by converting cell wall shape and charge 34. In normal cases, PC is stable at the outer layer of the cell membrane and PS is embedded inside the membrane. However, reciprocal transformation between PC and PS may occur and serve as a marker for cytotoxic drug use or drug abuse after long term induction. Previous studies have shown that Dox treatment was concomitant with decrease of enzymatic or non-enzymatic antioxidant enzyme levels 35. It is worth mentioning that the main side effects of Dox treatment are cardiotoxicity; in fact, nitric oxide is one of them. Several trials have been evaluated and have established that a mechanism of Dox-associated toxicity is cardiotoxicity 36. Currently, the most accepted reason is injury of myocardial cells by free radicals occurring after its metabolism. Nitric oxide oversecretion after Dox induction can lead to stimulation of free radicals which may induce cardiac failure, ischemia/perfusion injury, and cardiomyopathy. Free radicals, especially NO, is an intense vasodilator and huge contributor in myocardial contraction and in the pathogenesis of cardiac diseases. Indeed, an increase of NO in testicular cells disrupts all reproductive functions 37. Additionally, Dox affects the number of germ cells, induces atrophy of Leydig cells, and decreases the rate of spermatogenesis. Previous reports have confirmed that Dox can induce deterioration of sperm motion, sperm content and sperm morphology, which are responsible for the side effects on male fertility, due to ROS oversecretion 38. Flavonoids are a class of polyphenolic mixes which show an assortment of pharmacological activities. There has been recent interest in using flavonoid derivatives therapeutically as anticancer drugs. At pharmacological levels, various naturally occurring flavonoids have been shown to be cancer-protective in a variety of animal models 39. Quercetin and Apigenin are chemical molecules belonging to the flavonoid group, in particular, the flavonol family. Quercetin is one of the important molecules known to supplement vitamin C; it possesses anti-oxidant, anti-inflammatory, and anti-allergic properties 40. The data obtained data in our study showed an increase in the endogenous antioxidant system (GSH), a decrease in oxido-nitrosative markers (MDA and NO), a decrease in inflammatory markers (e.g. AA), and an increase in energy (ATP) after fifteen days of treatments. Furthermore, the rat group treat with CC showed cell membrane protection, as represented by stabilization of lipids and phospholipids; there was an increase of total lipids, phospholipids, and PC, and a decrease in PS, compared to the Dox-treated group. Quercetin and Apigenin are oxidized by radicals, resulting in more stability, which decreases radical reactivity, especially in ROS (through reacting with the reactive compounds of the radicals). The main effects of Quercetin and Apigenin may reach hydroxyl groups which induce inactive radicals, according to the following equation:
Flavonoid (OH) + R• > flavonoid (O•) + RH; whereby R• is a free radical, and O• is an oxygen free radical.
Stabilizing the lipid and phospholipid profile of testicular cells, and repairing the PC/PS ratio, as a result of scavenging highly reactive oxygen-derived radicals (e.g. peroxynitrite, ROS), propagated after Dox treatment 41. According to the oxidative stress markers, inflammatory mediators, endogenous antioxidant and testicular cell energy, Quercetin ameliorated the most negative symptoms from Dox induction. Nitric oxide produced by endothelial cells or macrophages, and the higher concentrations produced by inducible nitric-oxide synthase in macrophages, can result in oxidative damage. Flavonoids, thereby, ameliorate NO radicals which produce the mostly irreversible damage to the cell membrane. Our obtained data is in agreement with results by van Acker et al. 42, which showed NO as a radical that can be directly scavenged by flavonoids. The authors also demonstrated that Apigenin represses the development of prostate cancer cells by causing cell cycle capture and apoptosis. This is similar to results observed by Shukla et al. 32, who showed that Apigenin (at 6 to 64 mg/day) may inhibit the movement of prostate cancer cells in a transgenic adenocarcinoma mouse prostate (TRAMP) model. Furthermore, our data is in agreement with data from a study by Van et al. 43, who reported that Apigenin initiates FoxO3a and its DNA restriction can prompt the declaration of downstream target proteins (BIM and p27/Kip1), bringing about cell cycle capture and diminished expansion in prostate tumors. Apigenin could be used as a potential preventive and therapeutic agent in the management of prostate cancer in humans. Indeed, mice treated with low and high dose of Apigenin (20 and 50 mg/day, respectively) with the middle Apigenin dose group (40-50 mg/day), which is the equivalent administration of a dose of f120 mg/d of Apigenin in a grown-up. Taken together, our investigation supports the idea that Apigenin may confer a wide range of natural and chemopreventive effects on cells by influencing various cell cycle progression pathways or apoptotic cascades 44. Hung et al. 45 stated that Quercetin has a synergetic chemopreventive efficacy, when combined with Kaempferol, via the arrest of HCT-116 cells due to inhibition of DNA synthesis. As for the cascade pathway, Quercetin induces loss of HCT-116 viability and subsequently supports the structure/activity of 3’–OH moiety and/or 4’–OH moiety.
Conclusions
In conclusion, the data obtained in this study show the anticancer activity of Apigenin and Quercetin (constituents of CC) since they inhibited the growth and activity of human PC-3 prostate cancer and human HCT-116 colon cancer cell lines. The whole extract of CC led to an approximate convalescence from the reproductive side effects (induced by Dox treatment); this recovery was due to the flavonoids of the mixture (which were separated by HPLC). Thus, CC is cytotoxic against PC-3 and HCT-116 carcinoma cell lines and, moreover, the whole extract of CC ameliorates testicular dysfunction induced by Dox.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CCBY4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
AA: arachidonic acid; AQ: Apigenin and Quercetin; CC: Cymbopogon citratus; Dox: Doxorubicin; GSH: glutathione; H&E: Hematoxylin and eosin; HPLC: High performance liquid chromatography; MDA: malondialdehyde; NO: Nitric oxide; NODCAR: National Organization for Drug Control and Research; SRB: Sulfo-Rhodamine-B
Ethics approval and consent to participate
The protocol for the conducted animal experiments was approved by the Research Ethics Committee of the NODCAR which followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Competing interests
The authors declare that they have no conflicts of interest.
Authors' contributions
All authors contributed equally in the study design, interpretation of the data and writing of the final manuscript.
Acknowledgments
This study was performed in animal house of national organization of drug control and research (NODCAR), so, the authors thank animal house and Laboratory of Molecular Drug Evaluation and Physiology Department of NODCAR, Giza, Egypt.
References
-
Jalali
A. S.,
Hasanzadeh
S.,
Malekinejad
H..
Crataegus monogyna aqueous extract ameliorates cyclophosphamide-induced toxicity in rat testis: stereological evidences. Acta Medica Iranica.
2012;
50
:
1-8
.
-
Mikkola
M.,
Sironen
A.,
Kopp
C.,
Taponen
J.,
Sukura
A.,
Vilkki
J..
Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reproduction in Domestic Animals.
2006;
41
:
124-8
.
-
Rayburn
E. R.,
Ezell
S. J.,
Zhang
R..
Anti-Inflammatory Agents for Cancer Therapy. Molecular and Cellular Pharmacology.
2009;
1
:
29-43
.
-
da Cunha
M. F.,
Meistrich
M. L.,
Haq
M. M.,
Gordon
L. A.,
Wyrobek
A. J..
Temporary effects of AMSA (4′-(9-acridinylamino) methanesulfon-m-anisidide) chemotherapy on spermatogenesis. Cancer.
1982;
49
:
2459-62
.
-
Ruszniewski
P.,
Malka
D..
Hepatic arterial chemoembolization in the management of advanced digestive endocrine tumors. Digestion.
2000;
62
:
79-83
.
-
Petersen
P. E..
Oral cancer prevention and control—the approach of the World Health Organization. Oral Oncology.
2009;
45
:
454-60
.
-
Tacar
O.,
Sriamornsak
P.,
Dass
C. R..
Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems“. The Journal of Pharmacy and Pharmacology.2013; 65 (2): 157–70. Frederick CA, Williams LD, Ughetto G. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry.
1990;
29
:
2538-49
.
-
Frederick
Christine A,
Williams
Loren Dean,
Ughetto
Giovanni,
Van der Marel
Gijs A,
Van Boom
Jacques H,
Rich
Alexander,
Wang
Andrew HJ.
Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry.
1990;
29
(10)
:
2538-2549
.
-
Sonawane
A. C.,
Chaudhari
R. T.,
Bafna
Y. D.,
Kulkarni
M. N.,
Ram Mridula
B.,
Thorat
S. R..
Assessment of medicinal plant Cymbopogon citratus in north Maharashtra Univresity campus of Khandesh region. Current World Environment.
2008;
3
:
269-72
.
-
Tavares
F.,
Costa
G.,
Francisco
V.,
Liberal
J.,
Figueirinha
A.,
Lopes
M. C..
Cymbopogon citratus industrial waste as a potential source of bioactive compounds. Journal of the Science of Food and Agriculture.
2015;
95
:
2652-9
.
-
Boukhatem
M. N.,
Ferhat
M. A.,
Kameli
A.,
Saidi
F.,
Kebir
H. T..
Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. The Libyan Journal of Medicine.
2014;
9
:
25431
.
-
Bharti
S. K.,
Kumar
A.,
Prakash
O.,
Krishnan
S.,
Gupta
A. K..
Essential Oil of Cymbopogon Citratus Against Diabetes: Validation by In vivo Experiments and Computational Studies. Journal of Bioanalysis & Biomedicine.
2013;
5
:
194-203
.
-
Pulukuri
S. M.,
Gondi
C. S.,
Lakka
S. S.,
Jutla
A.,
Estes
N.,
Gujrati
M..
RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. The Journal of Biological Chemistry.
2005;
280
:
36529-40
.
-
Rajput
Ashwani,
San Martin
Ivan Dominguez,
Rose
Rebecca,
Beko
Alexander,
LeVea
Charles,
Sharratt
Elizabeth,
Mazurchuk
Richard,
Hoffman
Robert M,
Brattain
Michael G,
Wang
Jing.
Characterization of HCT116 human colon cancer cells in an orthotopic model. Journal of Surgical Research.
2008;
147
:
276-281
.
-
Feresin
G. E.,
Tapia
A.,
Gutierrez R
A.,
Delporte
C.,
Backhouse Erazo
N.,
Schmeda-Hirschmann
G..
Free radical scavengers, anti-inflammatory and analgesic activity of Acaena magellanica. The Journal of Pharmacy and Pharmacology.
2002;
54
:
835-44
.
-
Skehan
P.,
Storeng
R.,
Scudiero
D.,
Monks
A.,
McMahon
J.,
Vistica
D..
New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute.
1990;
82
:
1107-12
.
-
De Simon
F.,
Perez-Jlzarbe
B.,
Heranadez
J.,
Estrella
I..
HPLC study of the efficiency of extraction phenolic compounds. Chromatographia.
1990;
30
:
35-7
.
-
Dimitrios
K. P.,
Constantin
E.,
Harrala
C..
Achemometric comparison of three taxa of Scabiosa L.S. 1. Plant Biosystems.
2000;
134
:
67-70
.
-
Koh
P. H.,
Mokhtar
R. A.,
Iqbal
M..
Antioxidant potential of Cymbopogon citratus extract: alleviation of carbon tetrachloride-induced hepatic oxidative stress and toxicity. Human and Experimental Toxicology.
2012;
31
:
81-91
.
-
Alkreathy
H.,
Damanhouri
Z. A.,
Ahmed
N.,
Slevin
M.,
Ali
S. S.,
Osman
A. M..
Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food and Chemical Toxicology.
2010;
48
:
951-6
.
-
Papadoyannis
L. N.,
Samanidou
V. F.,
Nitsos
C. C..
Simultaneous determination of nitrite and nitrate in drinking water and human serum by high performance anion-exchange chromatography and UV detection. 1990;
22
:
2023-41
.
-
Karatepe
M..
Simulatenous determination of ascorbic acid and free malondialdehyde in human serum by HPLC-UV. 2004;
12
:
362-5
.
-
Abd-Elrazek
A. M.,
Ahmed-Farid
O. A..
Protective effect of L-carnitine and L-arginine against busulfan-induced oligospermia in adult rat. Andrologia.
2018;
50
:
e12806
.
-
Yassina
A.,
Hassan
A. M.,
Ahmed-Farid
O. A.,
Marwa
M..
Dromedary milk exosomes as mammary transcriptome nano-vehicle: their isolation, vesicular and phospholipidomic characterizations. 2016;
7
:
749-56
.
-
Ren
J.,
Mozurkewich
E. L.,
Sen
A.,
Vahratian
A. M.,
Ferreri
T. G.,
Morse
A. N..
Total Serum Fatty Acid Analysis by GC-MS: Assay Validation and Serum Sample Stability. Current Pharmaceutical Analysis.
2013;
9
:
331-9
.
-
Hassab El-Nabi
S. E..
Molecular and cytogenetic studies on the antimutagenic potential of eugenol in human lymphocytes culture treated with depakine and apetryl drugs. 2004;
43
:
171-96
.
-
Awwad
M. H..
Molecular identification of Biomphalaria alexandrai and Bulinus truncates using PCR-RFLP of Actin gene. 2003;
3
:
39-52
.
-
Coskun
O.,
Yakan
B.,
Oztas
E.,
Sezen
S.,
Gunaydin
A. A..
Antioxidant and hepatoprotective activity of vitamin E and EGb 761 in experimental endotoxemic rats. Turkish Journal of Medical Sciences.
2000;
30
:
427
.
-
Duncan’s
D..
Gives details of the test procedures and explains the reasons for using modified significance levels. 1955;
11
:
1-42
.
-
Das
Sreemanti,
Das
Jayeeta,
Paul
Avijit,
Samadder
Asmita,
Khuda-Bukhsh
Anisur Rahman.
Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. Journal of acupuncture and meridian studies.
2013;
6
(5)
:
252-262
.
-
Skandhan
K. P.,
Rajahariprasad
A..
The process of spermatogenesis liberates significant heat and the scrotum has a role in body thermoregulation. Medical Hypotheses.
2007;
68
:
303-7
.
-
Shukla
S.,
Bhaskaran
N.,
Babcook
M. A.,
Fu
P.,
Maclennan
G. T.,
Gupta
S..
Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis.
2014;
35
:
452-60
.
-
Yang
F.,
Teves
S. S.,
Kemp
C. J.,
Henikoff
S.,
Henikoffa
S..
Doxorubicin, DNA torsion, and chromatin dynamics. Biochimica et Biophysica Acta.
2014;
1845
:
84-9
.
-
Nakamura
K.,
Sugumi
H.,
Yamaguchi
A.,
Uenaka
T.,
Kotake
Y.,
Okada
T..
Antitumor activity of ER-37328, a novel carbazole topoisomerase II inhibitor. Molecular Cancer Therapeutics.
2002;
1
:
169-75
.
-
Kosoko
A. M.,
Olurinde
O. J.,
Akinloye
O. A..
Doxorubicin induced neuro- and cardiotoxicities in experimental rats: protection against oxidative damage by Theobroma cacao Stem bark. Biochemistry and Biophysics Reports.
2017;
10
:
303-17
.
-
Koleini
Navid,
Kardami
Elissavet.
Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget.
2017;
8
:
46663
.
-
Schug
T. T.,
Janesick
A.,
Blumberg
B.,
Heindel
J. J..
Endocrine disrupting chemicals and disease susceptibility. The Journal of Steroid Biochemistry and Molecular Biology.
2011;
127
:
204-15
.
-
Agarwal
A.,
Virk
G.,
Ong
C.,
du Plessis
S. S..
Effect of oxidative stress on male reproduction. The World Journal of Men\'s Health.
2014;
32
:
1-17
.
-
Ujiki
M. B.,
Ding
X. Z.,
Salabat
M. R.,
Bentrem
D. J.,
Golkar
L.,
Milam
B..
Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Molecular Cancer.
2006;
5
:
76
.
-
Li
Y.,
Yao
J.,
Han
C.,
Yang
J.,
Chaudhry
M. T.,
Wang
S..
Quercetin, Inflammation and Immunity. Nutrients.
2016;
8
:
167
.
-
Psotová
J.,
Chlopcíková
S.,
Miketová
P.,
Hrbác
J.,
Simánek
V..
Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part III. Apigenin, baicalelin, kaempherol, luteolin and quercetin. Phytotherapy Research.
2004;
18
:
516-21
.
-
van Acker
S. A.,
Tromp
M. N.,
Haenen
G. R.,
van der Vijgh
W. J.,
Bast
A..
Flavonoids as scavengers of nitric oxide radical. Biochemical and Biophysical Research Communications.
1995;
214
:
755-9
.
-
Shukla
S.,
Gupta
S..
Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Molecular Carcinogenesis.
2004;
39
:
114-26
.
-
Shukla
S.,
Gupta
S..
Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Molecular Cancer Therapeutics.
2006;
5
:
843-52
.
-
Tiong
H. K.,
Muriana
P. M..
RT-qPCR Analysis of 15 Genes Encoding Putative Surface Proteins Involved in Adherence of Listeria monocytogenes. Pathogens (Basel, Switzerland).
2016;
5
:
60
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 7 (2018)
Page No.: 2466-2479
Published on: 2018-07-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6386 times
- Download PDF downloaded - 1626 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress






