Abstract
Background: Acute ischemic stroke is caused by blockage of a cerebral artery and is also known as cerebrovascular accident (CVA). Recombinant tissue plasminogen activator (rt-PA) therapy is effective in reducing early and long-term neurologic disabilities if it is started quickly. Therefore, the aim of this study was to determine the efficacy of treatment with recombinant tissue plasminogen activators in improving the clinical status of acute ischemic stroke.
Methods: The current study was performed as a clinical trial of two groups- treatment and control (n=20 per group). The treatment group consisted of patients who received rt-PA, while the control group consisted of patients who did not receive rt-PA. For each group, evaluation of neurological disabilities following ischemic stroke was based off the National Institutes of Health Stroke Scale (NIHSS), for early assessment of disability on the third day of treatment), and off the modified Rankin Scale (MRS) at 90 days after stroke. The drug effect criterion was used to reduce the neurological disability or the difference in NIHSS on day 3 after treatment. Also, the duration of onset of symptoms until the arrival of the patients to the emergency room (ER), as well as the risk factors, complications and number of deaths in both groups, were recorded. Data obtained were analyzed by SPSS software.
Results: The results of the study showed that the mean of ER arrival time, NIHSS before treatment, and NIHSS on day 3 of treatment in the control group was higher than that of the treatment group; the difference was statistically significant (P<0.05). The results also indicated that the probability of improvement of neurological disabilities in the experimental group was greater than that of the control group (relative risk (RR) =1.25). Additionally, the odds ratio (OR) for receiving rt-PA in the NIHSS positive treatment group compared to the control group was equal to 1/5 (OR=1.5). The results showed that 30% of the patients in the treatment group were treated with a complication. The mean of MRS was higher in the control group at 90 days after the stroke, compared with the treatment group.
Conclusion: Treatment with rt-PA reduces the neurological disability in patients with ischemic stroke, since the mean of MRS is lower in the treatment group, compared with the control group, after 90 days of treatment.
Introduction
Stroke is a medical emergency caused by blockade or reduction of blood flow to the brain tissue. Stroke is the second leading cause of death worldwide, with around 55 million deaths each year, of which about 10% are due to stroke 1. The disease has a vascular origin and lasts more than 24 hours. It is a major cause of physical disability throughout the world and is the main cause of long-term hospitalizations, which can significantly increase the cost of treatment 2. Risk factors associated with the disease include increased age, gender, high blood pressure, diabetes mellitus (DM), smoking, obesity, low triglycerides, high cholesterol diet, high salt diet, alcohol consumption, family history, atrial fibrillation, and oral contraceptive pills (OCP) 345. The most common type of stroke is ischemic stroke, which consists of 85% of all strokes and is divided into two types (thrombotic and embolic) 6.
Since 1950, with the introduction of effective therapies for blood pressure, there has been a significant reduction in the incidence of stroke. However, with the increase in age, the death rate from stroke is expected to double by 2030.
In the United States, nearly 7800,000 cases of stroke occurred annually, of which 87% are ischemic and only 13% are hemorrhagic. Also, it leads to 150000 deaths in the United States. It is estimated that one of each 16 deaths which occurs in the United States, is due to stroke. While few studies in Iran have been conducted in this field, it has been reported that the prevalence of stroke in Iran is 43 cases per 100,000 people 789.
Indeed, stroke is one of the most common causes of mortality and disability worldwide; more than 80% of all patients are diagnosed with ischemic stroke 910. Considering the high mortality and morbidity of cerebral stroke, treatments for this disease are crucial. Therapies are targeted at regenerating blood flow, and can significantly improve the mortality and morbidity in patients with the appropriate anatomy of arteries and suitable conditions.
In most of the world's healthcare centers, patients with acute myocardial infarction are treated with agents, such as recombinant tissue plasminogen activator (rt-PA), that facilitate restoration of blood flow. Treatment of acute ischemic stroke with rt-PA is effective in decreasing disability when it is prescribed in the first 3 hours after onset of ischemic stroke. Now, rt-PA treatment is suggested as the only safe and effective treatment for cerebral ischemia 210.
The present objective of this study was to determine the efficacy of treatment with rt-PA in improving the clinical status of acute ischemic stroke.
Methods
Patients
This study was a clinical trial (code of ethics: IRCT201709049014N182) which was open to patients with acute ischemic stroke and whose medical records were referred to the Neurology Department of Farshchian Sina Hospital in Hamedan. The patients were admitted to the study from the beginning of April 2016 to the beginning of June Year 2017, and met the inclusion criteria.
The sample size in this study was as follows: 20 subjects in the treatment (experimental) group and 20 subjects in the control group. The control group was comprised of patients who were not eligible for treatment, based on candidate withdrawal criteria. Both groups were selected from patients who had been referred to the ER. To control confounding factors, the two groups were identical in terms of age, gender, and type of disease (all with stroke). The control group was selected from patients with acute ischemic stroke in the Neurology Department who did not undergo rt-PA injection.
Treatment Agent
The treatment group received tissue plasminogen activator (t-PA), a serine protease with corresponding gene in chromosome 8 (8P12) in humans. This protease catalyzes the transformation of plasminogen to plasmin, a major enzyme responsible for breaking down clots. Thus, it functions in decomposing clots containing fibrin, such as clots in cerebral thrombotic vascular lesions 11.
Patients with suspected symptoms were visited by a primary care physician in the ER. In case of symptoms indicating acute ischemic stroke, neurology services were introduced and, if they had the criteria for entering the study, they were enrolled to the study.
Ethical considerations
In the process of collecting, recording and analyzing the final report, the confidentiality of patient information was maintained.
Inclusion criteria
The inclusion criteria included: age of 18 years and older, clinical diagnosis of acute ischemic stroke, measurable neuromuscular failure, and onset of symptoms at least 180 minutes before the onset of treatment.
Exclusion criteria
Based on the information obtained from the history of the disease and physical examination, computed tomography (CT) scans and evaluations of the brain were carried out. The study exclusion criteria were as follows: use of heparin within 48 hours and impaired partial thromboplastin time (PTT), use of warfarin and international normalized ratio (INR) > 1.7, severe stroke within the past 3 months, gastrointestinal bleeding or thromboembolism during the past 3 weeks, major surgery in the past 2 weeks, arterial puncture at an inaccessible compressive site during the week before, previous intracerebral hemorrhage (ICH) at any time, brain tumor, arteriovenous malformation (AVM), cerebral aneurysm, seizures at the onset of symptoms, pregnancy, self-improvement of neurological symptoms, evidence of active bleeding during examination, presence of fractured, isolated or mildly defective nerves, non-debilitating clinical manifestations similar to subarachnoid hemorrhage (SAH), systolic hypertension above 185 or diastolic over 110, glucose lower than 50, platelets below 100,000, hyper-density indicating bleeding, hypo-density of more than one-third of brain hemisphere, and age over 80 years old.
Experimental design and treatment
Patient demographic data were completed by questionnaire. To evaluate the conditions for rt-PA injection, the patients were subjected to initial CT scan testing. The patients were then transferred to the intensive care unit (ICU). All injections were performed from the onset of the patient's symptoms until ICU arrival, within 4.5 hours to allow the patient to receive the injection. In the ICU, patients in the treatment group were infused with rt-PA (0.9 mg/kg to max 90 mg/kg, 10% bolus) over a 60-min period. The patients were then observed for signs of intracerebral hemorrhage. Determination of the response rate to the treatment at post-injection hours was conducted using the National Institutes of Health Stroke Scale (NIHSS). Another criterion for determining the outcome of the treatment group (after 90 days), compared to the control group, was the modified Rankin Scale (MRS).
Study Limitations
Some limitations of this study were that the start time of the stroke process was obscure, it was challenging to decide on when to start rt-PA treatment, and the examinations of neurological disorders were evaluated by different individuals in the study.
Statistical analysis
Data analysis was performed using SPSS software version 16. Quantitative data were shown (mean, standard deviation, frequency ratios, etc.), as well as qualitative data (tables, graphs, etc.).
Results
The results of the study showed that all patients in the treated (case) group and the untreated (control) group had risk factors for ischemic stroke. The highest risk factor in the treatment and control groups was high blood pressure (55%). The history of cardiovascular disease (CVD) in the treatment and control groups was estimated as 50%. More than one risk factor was reported in about 50% of the treatment group and 65% of the control group.
CVD/Smoking was estimated as 5% in the treatment group and nil in the control group. Moreover, DM/Smoking was estimated as 15% in the control group as compared to nil in the treatment group (Table 1).
| Variable | Frequency (%) | ||
| Treatment group (n=20) | Control group (n=20) | Total (n=40) | |
| High Blood pressure | 11(55) | 11(55) | 22(55) |
| CVD | 10(50) | 10(50) | 20(50) |
| DM | 6(30) | 7(35) | 13(32) |
| Smoking | 2(10) | 4(20) | 6(15) |
| More than 1 risk factor | 10(50) | 13(65) | 23(57) |
| CVD/DM | 2(10) | 3(15) | 5(12.5) |
| CVD/HTN | 3(15) | 4(20) | 7(12.5) |
| CVD/Smoking | 1(5) | 0(0) | 1(2.5) |
| HTN/DM | 3(15) | 0(0) | 3(7.5) |
| HTN/CVD/CVA | 1(5) | 2(10) | 3(7.5) |
| DM/Smoking | 0(0) | 3(15) | 3(7.5) |
| HPL/HTN | 0(0) | 1(5) | 1(2.5) |
The results of the study showed that the mean age in the treatment group and control group were not significantly different. However, the mean of ER arrival time, NIHSS before treatment, and NIHSS on the 3rd day of treatment for the treatment group were higher than those for the control group; the differences were statistically significant (P<0.05) (Table 2).
| Variable | Mean | P-value | |
| Treatment group (n=20) | Control group (n=20) | ||
| Age | 72.5 | 76.1 | 0.3 |
| Time to reach ER | 89.8 | 331.7 | 0.001 |
| NIHSS before treatment | 10.8 | 16.9 | 0.011 |
| NIHSS on the 3rd day of treatment | 9.6 | 9.6 | 0.009 |
The results of the study showed that in the rt-PA treatment group, the difference between the mean Pre-NIHSS-Post-NIHSS was 1.2, which indicates a trend of decrease in neurological disability at day 3 of treatment compared to pre-treatment period; however, this difference was not statistically significant (P>0.05). In the control group, the difference for Pre-NIHSS-Post-NIHSS was -0.05, which indicated that most of the neurological disabilities might occur on/after day 3 of treatment but this difference, too, was not statistically significant (P> 0.05) (Table 3).
| Variable | Difference in Mean | %95 CI | P-value | |
| Pre-NIHSS- Post-NIHSS | Treatment group | 1.2 | (-0.2, 2.6) | 0.09 |
| Control group | -0.5 | (-1.29, 1.19) | 0.9 |
Our findings showed that probability of recovery of neurologic disabilities in the experimental group is higher than in the control group (RR = 1.25) and also odd ratio for receiving rt-PA in the group which had positive post-treatment NIHSS - pre-treatment NIHSS compared with the control group was larger than one. It indicates that the odds of getting treatment in the group with positive Pre-treatment NIHSS-Post-treatment NIHSS was more than the control group (OR = 1.5).
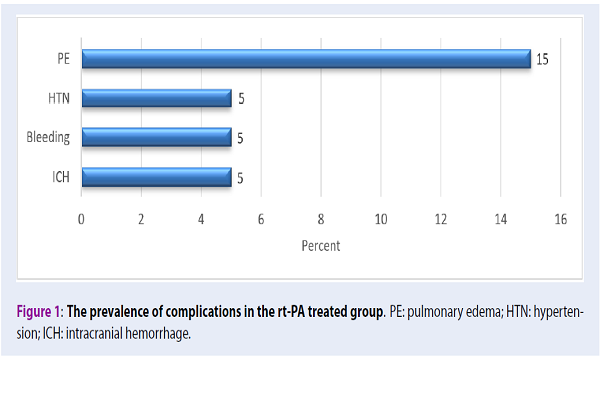
Furthermore, the results showed that among the 20 patients in the treatment group, 6 (30%) patients experienced complications of lung edema (15%), high blood pressure (5%), systemic hemorrhage (5%), as well as an intracranial hemorrhage (Figure 1).
In general, the mortality rate was estimated as 13 cases in both groups, 7 cases in the treatment group, and 6 in the control group. There was no significant difference between the treatment and control groups in terms of mortality (P=0.5).
Discussion
In recent years, improvements have been made in the treatment of acute ischemic stroke. One of the most important points to emphasize is that in the event of symptoms of ischemic stroke, the patient should immediately be sent to a facility equipped with the necessary facilities to start medical treatment. When the time interval between onset of symptoms and the onset of treatment is decreased, the less the effect of disability from the illness and, hence, a better prognosis.
Trials designed by National Institute of Neurological Disorders and Stroke (NINDS) showed that acute ischemic stroke treatment using rt-PA is effective in reducing disability, if it is administered 3 hours later. The use of intravenous rt-PA, nowadays, is considered to be an essential component of primary stroke centers. In fact, it is the first treatment that has been proven to improve clinical outcomes in ischemic stroke. In reviewing the efficacy of rt-PA in 1995, NINDS showed that intravenous rt-PA was associated with a 30% (15% -11%) decrease in relative risk 11.
In the current study, we show that treatment with rt-PA reduces the neurological disability in patients with ischemic stroke. The mean of the onset of symptoms to time of arriving in the ER was significantly lower in the experimental (rt-PA treatment) group than in the control group.
The mean of NIHSS can reflect the severity of the neurologic disabilities resulting from ischemic stroke. In the treatment group, the NIHSS before treatment (“pre-treatment”) versus on the 3rd day after treatment (“post-treatment”) was lower than the NIHSS pre/post for the control group. The relative risk for NIHSS pre-treatment prevention was 1.25, indicating that the rate of improvement in neurological disability in the rt-PA group was greater than that in the other group. Also, odd ratio for receiving rt-PA in the group which had positive post-treatment NIHSS — pre-treatment NIHSS compared with the control group was equal to 1.5. It indicates that the odds of getting treatment in the group with positive Pre-treatment NIHSS-Post-treatment NIHSS was more than the control group.
Early recanalization after rt-PA injection greatly improves the outcome of patients. However, intravenous thrombosis with rt-PA accounts for about 50% of stroke cases that successfully canalize the arteries. The onset of stroke until treatment is probably an important factor determining the next outcome 1.
Some types of acute ischemic stroke treatments include venous thrombolytic therapy, arterial thrombolytic therapy, non-pharmacological therapy (mechanical embolectomy), surgery, and supportive and therapeutic interventions. Among the treatments reported, rt-PA injection has been considered as the most available therapy accessible at most neurological centers.
In one study, after excluding participants over the age of 80, as well as those with severe stroke and diabetic cases, significant findings were obtained from tissue plasminogen injection. Thus, greater accessibility to visits by a neurologist could increase the number of injections in the eligible patients and improving the status of stroke patients. The causes of non-injection might be owing to the lack of correct diagnosis, the lack of imaging facilities, the lack of recognition of the patient's surroundings, the lack of recognition of primary care physician, and the incorrect transferring of the patient 12.
Our study confirms the benefits of rt-PA treatment since treatment led to reduction of neurological disability in patients with ischemic stroke. Indeed, we showed that the mean of onset of symptoms (until arrival at the ER) was significantly lower in the treatment group than in the control group. Previous analysis by Marler et al. 13 and Hacke et al. 14 have shown that there is a direct relationship between time and effect of treatment.
In our study, the criteria for assessing the difference in NIHSS before and after treatment were the judgment criteria for the effectiveness of the drug, which was shown to be lower in the NIHSS-treated group than before treatment. In a study by Guettier et al. in 2016, neurological premature improvement at 1 hour and neurological improvement within the first 24 hours after treatment were defined by improving NIHSS by 40% of baseline. Of the 421 patients, 51% had a very early neurological recovery and 26% had a neurological early recovery 12.
In a study by Li et al. (2013), primary neurological healing and continuous neurological healing in the first 24 hours were strong predictors of favorable outcome for 3 months in patients with acute ischemic stroke undergoing thrombolysis 15. Also, in a 2010 study by Kazumi Kimura et al., the amount of premature recanalization from the onset of stroke to rt-PA injection in patients with acute ischemic stroke should begin as soon as possible 1.
There are challenges in using tPA, which include the risk of marked intracerebral hemorrhage and lack of efficacy in some high-risk groups in the community. Recent American data suggest that the incidence of stroke will more than double by 2050. Therefore, better training and coordination among physicians is necessary to optimize the use of rt-PA for all patients with stroke.
Conclusion
Treatment with rt-PA reduces the neurological disability in patients with ischemic stroke. The median MRS is lowered after 90 days in the treatment group, compared to the control group, thereby indicating a decrease in the long-term neurologic disorder in the treatment group. In light of the beneficial effects of rt-PA treatment in this study, it is recommended that patients with acute stroke be treated with rt-PA as soon as possible. The benefits of early injection of this agent should be known to all neurologists, general practitioners and ER physicians, who are in the first line of dealing with such patients.
Competing Interests
The authors declare that there is no conflict of interest.
Authors' Contributions
All authors contributed to the design of the research. AGH, EG, and MKH collected the data. ZKH, EG conducted analysis and interpretation of data. All authors drafted the first version. MKH, ZKH, EG, and AGH edited the first draft. All authors reviewed, commented and approved the final draft.
Financial support
This research is the result of a dissertation in the general population with a code identifier IRCT201709049014N182 from the Faculty of Medicine of Hamedan University of Medical Sciences, supported by Hamedan University of Medical Sciences.
Abbreviations
AVM: Arteriovenous Malformation
DM: Diabetes Mellitus
ICH: Intra-cerebral Hemorrhage
OCP: Oral Contraceptive Pills
RCT: Randomized Clinical Trial
SAH: Subarachnoid Hemorrhage
References
-
Kimura
K.,
Iguchi
Y.,
Shibazaki
K.,
Aoki
J.,
Watanabe
M.,
Matsumoto
N..
Early stroke treatment with IV t-PA associated with early recanalization. Journal of the Neurological Sciences.
2010;
295
:
53-7
.
PubMed Google Scholar -
Mouradian
M. S.,
Senthilselvan
A.,
Jickling
G.,
McCombe
J. A.,
Emery
D. J.,
Dean
N..
Intravenous rt-PA for acute stroke: comparing its effectiveness in younger and older patients. Journal of Neurology, Neurosurgery, and Psychiatry.
2005;
76
:
1234-7
.
PubMed Google Scholar -
Poorthuis
M. H.,
Algra
A. M.,
Algra
A.,
Kappelle
L. J.,
Klijn
C. J..
Female-and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurology.
2017;
74
:
75-81
.
PubMed Google Scholar -
Jokela
M.,
Pulkki-Råback
L.,
Elovainio
M.,
Kivimäki
M..
Personality traits as risk factors for stroke and coronary heart disease mortality: pooled analysis of three cohort studies. Journal of Behavioral Medicine.
2014;
37
:
881-9
.
PubMed Google Scholar -
Lightbody
C Elizabeth,
Clegg
Andrew,
Patel
Kulsum,
Lucas
Julie Cook,
Storey
Hannah,
Hackett
Maree L,
Watkins
Dame Caroline L.
Systematic Review and Meta-Analysis of Psychosocial Risk Factors for Stroke. Seminars in neurology.
2017;
37
:
294-306
.
-
Toni
D.,
Angelantonio
E. Di,
Mascio
M. T. Di,
Vinisko
R.,
Bath
P. M.,
Group
P. S.,
null
null.
Types of stroke recurrence in patients with ischemic stroke: a substudy from the PRoFESS trial. International Journal of Stroke.
2014;
9
:
873-8
.
PubMed Google Scholar -
Fang
J.,
Yuan
K.,
Ayala
C.,
Gindi
R.,
Ward
B..
2016.
Google Scholar -
Krueger
H.,
Koot
J.,
Hall
R. E.,
O’Callaghan
C.,
Bayley
M.,
Corbett
D..
Prevalence of individuals experiencing the effects of stroke in Canada: trends and projections. Stroke.
2015;
46
:
2226-31
.
PubMed Google Scholar -
Adeloye
D..
An estimate of the incidence and prevalence of stroke in Africa: a systematic review and meta-analysis. PLoS One.
2014;
9
:
e100724
.
-
Fugate
J. E.,
Rabinstein
A. A..
Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. The Neurohospitalist.
2015;
5
:
110-21
.
-
Chapman
S. N.,
Mehndiratta
P.,
Johansen
M. C.,
McMurry
T. L.,
Johnston
K. C.,
Southerland
A. M..
Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vascular Health and Risk Management.
2014;
10
:
75-87
.
-
Guettier
S.,
Cogez
J.,
Bonnet
A. L.,
Dean
P.,
Apoil
M.,
Tchoumi
T..
Factors associated with timing of early neurological improvement after thrombolysis for ischaemic stroke. European Journal of Neurology.
2016;
23
:
664-7
.
-
Marler
J. R.,
Tilley
B. C.,
Lu
M.,
Brott
T. G.,
Lyden
P. C.,
Grotta
J. C..
Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology.
2000;
55
:
1649-55
.
PubMed Google Scholar -
Hacke
Werner,
Donnan
Geoffrey,
Fieschi
Cesare,
Kaste
Markku,
Broderick
JP,
Brott
T,
Frankel
M,
Grotta
JC,
Haley
Jr EC,
Kwiatkowski
T.
Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. null.
2004
.
PubMed Google Scholar -
Yeo
L. L.,
Paliwal
P.,
Teoh
H. L.,
Seet
R. C.,
Chan
B. P.,
Wakerley
B..
Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. Journal of Stroke and Cerebrovascular Diseases.
2013;
22
:
e590-6
.
Comments
Downloads
Article Details
Volume & Issue : Vol 5 No 9 (2018)
Page No.: 2697-2702
Published on: 2018-09-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4222 times
- Download PDF downloaded - 1176 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



