
Effect of Vitamin D deficiency in lower extremity and pulmonary venous thromboembolism
- Department of Cardiology, Jahrom University of Medical Science, Jahrom, Iran
- Medical Student of Research Committee, Jahrom University of Medical Science, Jahrom, Iran
- Department of Surgery, Kerman University of Medical Science, Kerman, Iran
- Department of Internal Medicine, Jahrom University of Medical Science, Jahrom, Iran
Abstract
Introduction: Vitamin D deficiency increases inflammation and dysfunction of pancreas betacells, resulting in atherosclerotic disorders, cerebrovascular disorder, and CVDs.
Methods: In the present cross-sectional study, vitamin D was evaluated in the plasma of 42 patients with lower extremity DVT or PE, as well as 42 healthy controls. Using the chemiluminescence assay, the plasma vitamin D levels were determined. After collection, the blood samples were examined within 60 minutes. Vitamin D levels were classified as sufficient, insufficient, and deficient (> 30 ng/mL, 20- 29 ng/mL, and < 20 ng/mL, respectively).
Results: The prevalence of deficiency in vitamin D was higher in the cases than the controls. The two groups were significantly different regarding vitamin D levels (p = 0.024). Based on the vitamin D classification, deficiency was reported in 30 (71.4%) patients and 18 (42.9%) controls.
Conclusion: Our findings indicated that VTE patients had lower concentration of vitamin D, and the correlation between VTE and vitamin D deficiency was confirmed.
Introduction
Vitamin D is recognized as a major factor in human health, which includes preventing osteoporosis and osteomalacia in adults, as well as rickets in children. Vitamin D is traditionally known to decrease the risk of cardiovascular diseases (CVDs), cancers, and chronic disorders1,2,3,4. On the other hand, vitamin D deficiency increases inflammation and dysfunction of pancreas beta-cells, resulting in atherosclerotic disorders, cerebrovascular disorder, and CVDs 5,6,7,8.
Deficiency in vitamin D is characterized by a plasma 25-hydroxyvitamin D concentration lower than 20 ng/mL. It is described as a common global problem with a prevalence of 30-50% population 3,4, and is involved in CVDs and venous thromboembolism (VTE) 5,6,9,10,11. In leukemic patients, active vitamin D stimulates anticoagulant effects through tissue factor downregulation and thrombomodulin upregulation 12,13,14.
Vitamin D receptors are involved in thrombosis 15,16. On the other hand, VTE is recognized as a common CVD, resulting in morbidity and mortality17. Overall, the VTE spectrum, including deep vein thrombosis (DVT) and pulmonary embolism (PE), affects almost 2 per 1000 population 18. The correlation of vitamin D deficiency with thromboembolism has been highlighted in multiple studies 19,20. Based on previous findings, the risk of VTE is reduced with an increase in vitamin D levels 9. In addition, the use of vitamin D in patients with prostate cancer reduces the incidence of DVT9,20. Therefore, vitamin D deficiency increases inflammation and dysfunction of pancreas beta-cells, resulting in atherosclerotic disorders, cerebrovascular disorder, and CVDs. In this study, we aimed to evaluate an association of vitamin D levels, pulmonary and lower extremity venous thromboembolism.
Methods
Patients
In the present cross-sectional study, vitamin D was evaluated in the plasma of patients with lower extremity DVT or PE, as well as healthy controls (age- and sex-matched). The study sample included ICU and CCU patients with suspected DVT or PE, along with patients visiting Honari Clinic (Jahrom, Southwest of Fars Province, Iran) for a routine checkup from June 2016 to June 2017.
The case and control groups included 42 DVT and PE patients and 42 healthy individuals, respectively. The control group did not have any common risk factors for VTE. This study was approved by the Jahrom University of Medical Sciences. Also, for the collection of blood samples, all participants were asked to provide their written informed consents.
A combination of D-dimer test, clinical probability, spiral chest CT scan, and color Doppler ultrasound was applied for the diagnosis of DVT and PE in patients. The exclusion criteria were as follows: 1) pregnancy; 2) cancer; 3) hormone replacement therapy; 4) chronic renal disease (above stage II) or renal failure; 5) vitamin D supplementation in the last two years; 6) major trauma or surgery in the last three months; 7) family history of VTE, and 8) positive thrombophilia markers.
Finally, none of the participants was excluded. No signs or symptoms of VTE, VTE history, positive thrombophilia, or vitamin D supplementation were reported in the control group; also, they did not meet the mentioned exclusion criteria. Upon admission, demographic information such as history of hypertension, surgery, obesity, smoking, diabetes mellitus, dyslipidemia, and ischemic heart disease, was collected.
Chemiluminescence assay
Using the chemiluminescence assay, the plasma vitamin D level was determined. Blood samples were collected and examined within 60 minutes. The vitamin D level was classified as sufficient, insufficient, and deficient (> 30 ng/mL, 2-29 ng/mL, and < 20 ng/mL, respectively) 1.
Statistics analysis
For statistical analysis, SPSS 21.0 was used, and the significance p value was 0.05. For the evaluation of categorical variables, Chi square test was performed, while Mann–Whitney U test, Kruskal-Wallis, or student t-tests were applied for examining continuous variables.
Results
The mean age of the participants was 47.52±18.31 years, and the mean vitamin D concentration was 21.79±13.73. Overall, 61.9% were male and 38.1% were female. The prevalence of deficiency in vitamin D was higher in the case group than the controls. The two groups were significantly different regarding vitamin D levels ( = 0.024). Based on the vitamin D classification, deficiency was reported in 30 (71.4%) patients and 18 (42.9%) controls.
Comparison of age, sex, and BMI between the groups
| Variable | Case n=42 (%) | Control n=42 (%) | P-value | |
|---|---|---|---|---|
| Sex | Male | 26(61.9) | 26(61.9) | 0.241 |
| Female | 16(38.1) | 16(38.1) | ||
| BMI | Underweight | 1(2.4) | 0 | 0.99 |
| Normal | 20(47.6) | 22(52.4) | ||
| Overweight | 18(42.9) | 20(47.6) | ||
| Obese | 3(7.1) | 0 | ||
| Age | 30> | 7(16.7) | 11(26.2) | 0.55 |
| 30-60 | 25(59.5) | 23(54.8) | ||
| 60< | 10(23.8) | 8(19) |
The correlation of vitamin D level with demographic data and known risk factors for VTE in patients with DVT or PE
| Vitamin D Level | |||||
|---|---|---|---|---|---|
Risk Factors | Deficiency (%) | Inadequate (%) | Sufficient (%) | P-Value | |
| Diabetes Mellitus | Positive | 26(86.7) | 3(75) | 7(87.5) | 0.811 |
| Negative | 4(13.3) | 1(25) | 1(12.5) | ||
| Surgery History | Positive | 21(70) | 2(50) | 4(50) | 0.474 |
| Negative | 9(30) | 2(50) | 4(50) | ||
| Hyperlipidemia | Positive | 20(66.7) | 4(100) | 7(87.5) | 0.225 |
| Negative | 10(33.3) | 0 | 1(12.5) | ||
| Hypertension | Positive | 21(70) | 4(100) | 6(75) | 0.438 |
| Negative | 9(30) | 0() | 2(25) | ||
| Smoker | Positive | 23(76.7) | 3(75) | 6(75) | 0.993 |
| Negative | 7(23.3) | 1(25) | 2(25) | ||
| Predictive pill | Positive | 27(90) | 4(100) | 6(75) | 0.377 |
| Negative | 3(10) | 0 | 2(25) | ||
| Ischemic Heart Disease | Positive | 20(69) | 3(75) | 6(75) | 0.928 |
| Negative | 9(31) | 1(25) | 2(25) | ||
| Absolute Rest (More than 3 day) | Positive | 21(70) | 2(50) | 4(50) | 0.474 |
| Negative | 9(30) | 2(50) | 4(50) | ||
Demographic Factor | Deficiency (%) | Inadequate (%) | Sufficient (%) | P-value | |
| Sex | Male | 20(66.7) | 3(75) | 3(37.5) | 0.273 |
| Female | 10(33.3) | 1(25) | 5(62.5) | ||
| BMI | Underweight | 0 | 0 | 1(12.5) | 0.233 |
| Normal | 12(40) | 3(75) | 5(62.5) | ||
| Overweight | 15(50) | 1(25) | 2(25) | ||
| Obese | 3(10) | 0 | 0 | ||
| Age | 30> | 5(16.7) | 0 | 2(25) | 0.505 |
| 30-60 | 17(56.7) | 4(100) | 4(50) | ||
| 60< | 8(26.7) | 0 | 2(25) |
The data analysis in
Based on the Chi-square test, vitamin D was not linked to VTE risk factors, including diabetes, BMI, age, sex, history of surgery, hyperlipidemia, hypertension, smoking, the use of preventive pills, ischemic heart disease, and more than three days of absolute rest ( > 0.05).
Deficiency in vitamin D was clinically more frequent in male DVT or PE patients (66.7%), compared to female counterparts (33.3%). In addition, it was more common among patients with diabetes (13.3%), history of surgery (30.0%), hyperlipidemia (33.3%), hypertension (30.0%), smoking (23.3%), the use of preventive pills (10%), history of ischemic heart disease (31%), and patients with absolute rest in more than three days (30%). Vitamin D deficiency showed the highest rate in overweight cases (50%) and patients aged 30-60 years (56.7%) (Figure 1).
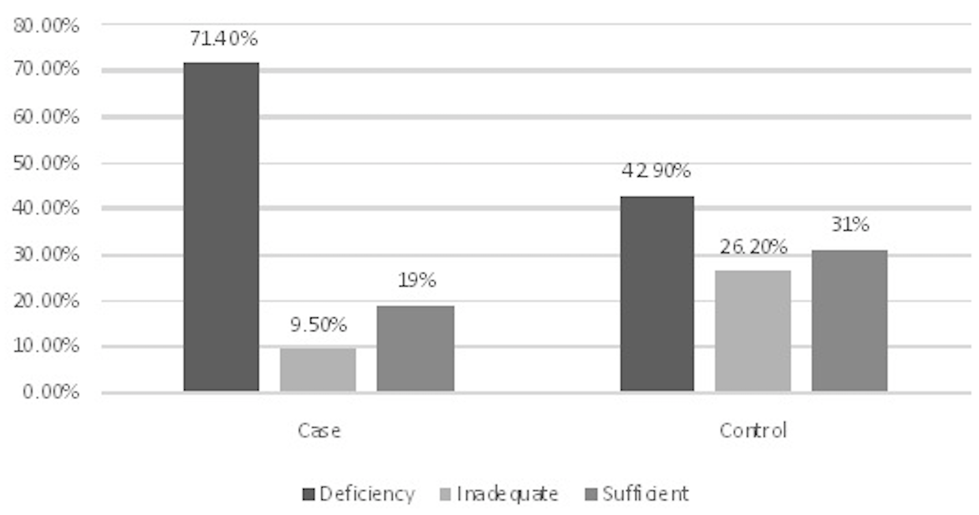
Comparison of the status of vitamin D levels among VTE patients and healthy population.
Discussion
VTE is recognized as the third most common CVD associated with mortality. According to a previous meta-analysis, vitamin D deficiency increased all-cause mortality besides CVD and cancer-related mortality 21.
Our findings indicated that VTE patients had lower concentrations of vitamin D, and the correlation between VTE and vitamin D deficiency was confirmed. The prevalence of deficiency in vitamin D was higher in the cases than the controls. The two groups were significantly different regarding vitamin D level ( = 0.024). Based on the vitamin D classification, deficiency was reported in 30 (71.4%) patients from the case group and 18 (42.9%) controls. The prevalence of vitamin D deficiency among male and female PTE patients were 66.7 and 33.3 %, respectively ( = 0.273). Overall, healthy female adults had more Vitamin D deficiency in Iranian population. This subject is in contrast with our study on PTE patients.
In the present study, hypertension was revealed in 70% of PTE patients with vitamin D deficiency ( = 0.438). Overall, deficiency in vitamin D is related to the risk of hypertension and CVDs based on observational studies 22, while most randomized trials have not reported any cardiovascular benefits from vitamin D supplements. However, the main reason for the association between CVDs and vitamin D levels in different patient populations remains uncertain20,23,24. According to another meta-analysis, the risk of CVDs was correlated inversely with serum vitamin D in 19 prospective studies 25. Moreover, in the Framingham Offspring Study, cardiovascular events were more significantly associated with vitamin D < 15 ng/mL (vs. > 15 ng/mL) during 5.4 years of observation 5.
As reported in a large survey during 2001-2004, vitamin D levels of less than 20 ng/mL contributed to a higher risk of coronary heart disease when compared to the levels of ≥ 30 ng/mL 26,27. Moreover, Entezari-Maleki 28 evaluated the risk factors for VTE, hs-CRP, and P-selectin in patients with acute DVT or PE. The study was conducted on 60 subjects with a vitamin D levels of 21.4±14.6 ng/mL, which was close to our study (21.79±13.73 ng/mL). They indicated the high frequency of vitamin D deficiency in Iranian VTE patients, which is consistent with our study. Nevertheless, vitamin D levels had no association with age, diabetes, or other risk factors for VTE.
Additionally, Khademvatani and colleagues 29 indicated a significantly lower serum levels of vitamin D in DVT patients in comparison with the controls. It was concluded that idiopathic lower extremity DVT was related to low vitamin D levels, which is consistent with our results. Ohsawa and colleagues 13 emphasized that tissue and thrombomodelin regulation by analogue 1, 25(OH) 2 D 3 was accomplished by vitamin D receptors, and these analogues could be used to treat or prevent atherosclerotic and thrombotic disorders. Therefore, the involvement of vitamin D in thrombosis development was confirmed, which is consistent with our study.
Brøndum-Jacobsen 10 evaluated the link between VTE and vitamin D among 18791 participants over 30 years. In this extensive study, the increased risk of VTE was attributed to a seasonal decline in vitamin D concentrations; this finding represented the link between VTE and vitamin D deficiency similarly to our study. Another study also reported congruent results with our study, which showed the contribution of vitamin D to non-age-related coagulability and BMI 30.
In 2004, Ken-ichi Aihara and colleagues explored the issue that breakage of the nuclear receptor of vitamin D would increase thrombogenesis in mice. Based on their findings, vitamin D receptor (VDR) activation induced antithrombotic effects. They also stated that VDR might be involved in the maintenance of antithrombotic homeostasis. The antithrombotic role of vitamin D was established in their study, which is consistent with the results of our investigation 16. In contrast, E Brodin indicated that serum vitamin D was not involved in the pathogenesis or risk of VTE; nevertheless, this finding calls for further research 31.
Generally, the prevalence of CVDs is rising, and it is one of the major causes of VTE and clot formation. Regarding the possible positive connection between CVDs and vitamin D, as reported in the limited literature, the exact correlation between vitamin D and VTE should be evaluated in further comprehensive studies.
This study had some limitations. There is increasing evidence in terms of seasonal effects on 25(OH)D levels and it has been demonstrated that vitamin D concentration is at its lowest in the winter months compared to summer. However, in our study we could not investigate the seasonal impact on DVT incidence because our study was conducted during the warm months. Prothrombin gene mutation was not assessed in this study. The small sample size of our study was another restriction.
Conclusions
This study showed that lower extremity and pulmonary venous thromboembolism patients had lower concentrations of vitamin D, and the correlation between VTE and vitamin D deficiency was confirmed.
Abbreviations
CVD: cardiovascular diseasesVTE: Venous ThromboembolismDVT: Deep Vein ThrombosisPE: Pulmonary embolismCCU: Cardiac Care UnitICU: Intensive Care UnitCT: Computed TomographyVDR: Vitamin D Receptor
CRP: C-Reactive Protein
Conflict of Interests
The authors declare that they have no conflicts of Interest.
Authors' Contributions
Khatere Dehghani: Design of the study, acquisition of data, and writing of the article. Aygin Nowrouzi: Collection of data and revision of the article. Amir Hossein Pourdavood: Analysis and interpretation of data and revision of the article. Zhila Rahmanian: Design of the study, Revision of the article and final approval of the version to be published.

