
Centella asiatica prevents chronic unpredictable mild stress-induced behavioral changes in rats
- Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
- 3Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Maiduguri, 600230 Maiduguri, Borno State, Nigeria
- Faculty of Health Sciences, Universiti Teknologi Mara (UiTM) Kampus Puncak Alam, 42300 Bandar Puncak Alam, Selangor, Malaysia
- Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknologi Mara (UiTM) Kampus Puncak Alam, 42300 Bandar Puncak Alam, Selangor, Malaysia
Abstract
Background: Depression is a psychological disorder which is associated with mood swings and cognitive deficits, and is ranked third among the leading causes of global health care burden. Chronic unpredictable mild stress (CUMS) has been shown to induce depression-like behaviors in rodents, which exhibit similarities to the human form of depression. Centella asiatica (CA) is an ancient medicinal plant which has neuroprotective potential. This work aimed to assess the protective role of CA on CUMS-induced rats.
Methods: Thirty six rats randomly divided into six groups were used for the evaluation. The groups were as follows: 1, Unstressed + normal saline (control); 2, CUMS + normal saline (model); 3, CUMS + fluoxetine (10 mg/kg) standard antidepressant; 4-6, CUMS + CA (200, 400 & 800 mg/kg). Nine different stressors (S) (S1: 24-h food deprivation, S2: 24- h water deprivation, S3: 5-min cold swimming (at 5◦C), S4: 12-h change of cage mate, S5: 1-min tail pinch (1 cm from the tip of the tail), S6: 12-h cage tilt (at 45◦), S7: 12-h overcrowding of cage, S8: 12-h wet bedding with 200 mL of water, and S9: 4-h physical restraint) were administered to the stressed groups with at least 2 stressors per day. The treatments lasted for 8 weeks, and the rats were evaluated through open field test (OFT) and elevated plus maze (EPM) for anxiety-like behavior, forced swimming test (FST) for depression-like behaviour, and T-maze spontaneous alternation for learning and memory. Furthermore, serum cortisol levels were also evaluated.
Results: CUMS-induced rats showed anxiety-like behaviors in OFT and EPM tests, depression-like behavior in FST and cognitive deficits in T-maze test, as well as increased serum cortisol levels. Conversely, administration of CA (at 400 and 800 mg/kg doses) and fluoxetine (at 10 mg/kg dose) prevented the aberrant behavioral changes and also the changes in the serum cortisol levels. No significant differences of behaviors were observed between the groups of rats administered with CA (400 and 800 mg/kg) and those administered with fluoxetine (10 mg/kg), suggesting that the therapeutic potential of CA is comparable to that of fluoxetine.
Conclusion: The data obtained showed that CA, at doses of 400 and 800 mg/kg, effectively reversed the anxiety and depression-like behaviors, amnesic behaviors, as well as serum cortisol levels in a CUMS-induced rat model of depression. This suggests that CA is a potential candidate for the development of future anti-depressants.
Introduction
Depression is a major public health challenge and is ranked third among the top three foremost causes of global health care burden globally1. Besides the mood disorders that are exhibited by depressed patients, cognitive impairments (such as difficulty in decision-making, memory deficits difficulty in learning, and loss of cognitive flexibility) have also been reported 2,3. Accumulating evidence have shown that these cognitive insufficiencies could be an early occurrence in depression and could help predict the probability of recovery 4.
Chronic stress is one of the significant contributory factors for various neurological disorders, such as depression and anxiety, and plays a key role leading to cognitive impairments in neurodegenerative diseases 5,6. The chronic unpredictable mild stress (CUMS) protocol is a classic method that has been used to induce depression-like behaviors and cognitive deficits in rat models and also to study the underlying mechanisms 7. Since the hippocampus is sensitive to CUMS, impairments of hippocampal structure and functions, such as mental and cognitive deficits, are exhibited by rats which are exposed to the CUMS protocol 8. Preclinical studies have shown decreased expression of brain-derived neurotrophic factor (BDNF), altered synaptic morphology, and reduced neurogenesis in the hippocampus of rats which were exposed to CUMS 9. Previous researches have shown that when rats are exposed to chronic restraint or chronic mild stress, they exhibit cognitive dysfunction, depletion of BDNF and cAMP-response element binding protein (CREB), and dendritic atrophy of the cornus ammonis 3 (CA3) sub-region of the hippocampus 10,11.
Centella asiatica (CA) (L.) Urban, of the family Apiaceae, also known as Brahmi or Mandookaparni in Ayurvedic medicine 12. It is also known as Indian pennyworth in the United States of America (USA), Gotu Kola in Indonesia and Pegaga in Malaysia 13. CA grows extensively in shady, marshy, damp and wet places, such as paddy fields and river banks 14. CA is a slender, creeping plant with rooting at the nodes and growing among the damp areas in tropical countries 15. CA is a faintly aromatic, creeper herb which grows perennially, attaining the height of 15 cm (6 inches) with a glabrous stem. The plant grows well on loamy soil 16. The reniform shaped leaves grow from the nodes on the stem and measure 1.5-5 cm in width and 2-6 cm in length, with flowers which appear as fascicled umbels 14.
In Ayurvedic medicine, CA is regarded as a rejuvenating herb and as a neurotonic known to increase intelligence and memory 12. In addition to its neuro-rejuvenating properties, CA is also used for its psychoactive medicinal properties, such as in the treatment of anxiety in Ayurvedic medicine 17. The triterpenoid glycosides, including madecassic acid, asiatic acid, madecassoside, and asiaticoside, are the most active compounds isolated from CA 18. The whole plant can be used for medicinal purposes, while the nutritive value remains the same for all parts of the plant 19 . As a nutritive supplement, CA has been used for the treatment of sleep disorders in patients with mental health problems. Further, 70% hydro-ethanolic extract of CA has shown to reduce stress phenomenon and attenuate anxiety-related disorders, as documented in a clinical study 20. Various preclinical studies to evaluate the properties of CA have demonstrated that it has antioxidant21, anti-acetylcholinesterase, and anti-apoptotic21,22 properties, as well as neuroprotective effects, besides amelioration of learning and memory deficits 23,24. While asiaticoside in the methanolic extract of CA has been demonstrated to have anxiolytic effects in acutely stressed animals 25,26 and in chronically immobilized stressed-induced anxiety models 27, no studies have been conducted as of yet to discern the effects of CA on anxiety, depression-like behaviors, and cognitive deficits in CUMS-induced rats.
The present study evaluated the anxiolytic, antidepressant, and anti-amnesic effects of CA on CUMS-induced rats, with reference to fluoxetine, an established antidepressant. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) which has high selectivity for the 5-hydroxytryptamine (5-HT) transporter, which helps to modulate the concentration of serotonin in the synapses 28. Fluoxetine interacts with ligands, such as channel ions (Na, K, and Ca) 29 and monoamine oxidases A and B (MAO-A and MAO-B) 30, and inhibits these proteins. Fluoxetine also affects both muscular and neuronal nicotinic receptors 31. Though widely used, fluoxetine has several side effects including anxiety, sexual dysfunction, sleep disturbances, and gastrointestinal impairments 32. The anxiolytic effects of CA were evaluated through open field test (OFT) and elevated plus maze (EPM); the antidepressant effects were assessed via forced swimming test (FST) while the anti-amnesic effects were evaluated by T-maze spontaneous alternation test.
Materials and methods
Drugs and chemicals
Fluoxetine (Cadila Pharmaceuticals Ltd, Bhat, Ahmedabad, India) and CA extract (Reference number: AuRins-MIA-1-0, Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknology Mara (UiTM) Puncak Alam, Selangor, Malaysia) 33, were acquired from the respective sources. The extraction method for CA was performed according to the methods described by Wong . 34. Drugs and treatments were administered in the morning between 9 a.m. to 11 a.m. The doses of fluoxetine (10 mg/kg) per dose (p.o.) (. oral administration) and CA (200, 400 and 800 mg/kg) p.o. were on the basis of previous studies 24,35. Fluoxetine and CA were administered orally because it was considered the best route of drug administration for psychiatric patients 35.
Animals
Thirty-six male albino Wistar rats, aged 8-10 weeks and weighing 180-220g, were purchased from a local supplier (Bistari Ltd, Serdang, Selangor, Malaysia) and were used for this study. The rats were kept in the Animal House, Faculty of Medicine and Health Sciences Universiti Putra Malaysia and were maintained under standard laboratory conditions (12:12 h light/dark cycle, light on at 0700 h, 25±2°C, relative humidity 50±10% and food and water ). The rats were allowed to acclimatize with the laboratory conditions for 1 week. All the non-stress exposed rats were kept 2 per cage while the stress exposed rats were kept singly or 2 per cage. The number of rats used and the protocol followed for the experiment was approved by the Institutional Animal Care and Use Committee, Universiti Putra Malaysia, on 23 November 2018, with project identification code UPM/IACUC/AUP-R078/2018.
CUMS procedure
CUMS was applied for 8 weeks. The rats were subjected to various psychosocial and environmental stressors, as previously described 5,36,37 with minor modifications. The rats of the control group were left in their home cages except during the usual general handling and cleaning process. The CUMS-induced and drug-treated groups of rats were exposed to 9 different mild stressors (S) with a minimum of 2 stressors per day for 8 consecutive weeks:
S1: 24-hours of food deprivation;
S2: 24-hours of water deprivation;
S3: 5-minutes of cold swimming (at 5°C);
S4: 12-hours change of cage mate;
S5: 1-minute tail pinch (1 cm from the tip of the tail);
S6: 12-hours cage tilt (at 45°);
S7: 12-hours overcrowding of cage;
S8: 12-hours of wet bedding with 200 mL of water; and
S9: 4-hours of physical restraint.
These stressors were randomly scheduled for 1 week and repeated throughout the experimental period. To avoid prophesy and adaptation, no single stressor was performed consecutively.
Experimental design
After one week of acclimatization, the 36 rats were randomly divided into six groups (G) (n=6):
G1, Unstressed + normal saline (control); G2, CUMS + normal saline (model); G3, CUMS + fluoxetine (10 mg/kg) standard antidepressant (Flx); and G4-6, CUMS + CA (200, 400 & 800 mg/kg). Fluoxetine and CA were administered starting from day zero of the experiment once a day for 8 weeks. Behavioral tests were conducted starting from the 9 week of the experiment, after 12 pm daily. The detailed schedule of the experimental procedures are presented in Figure 1.
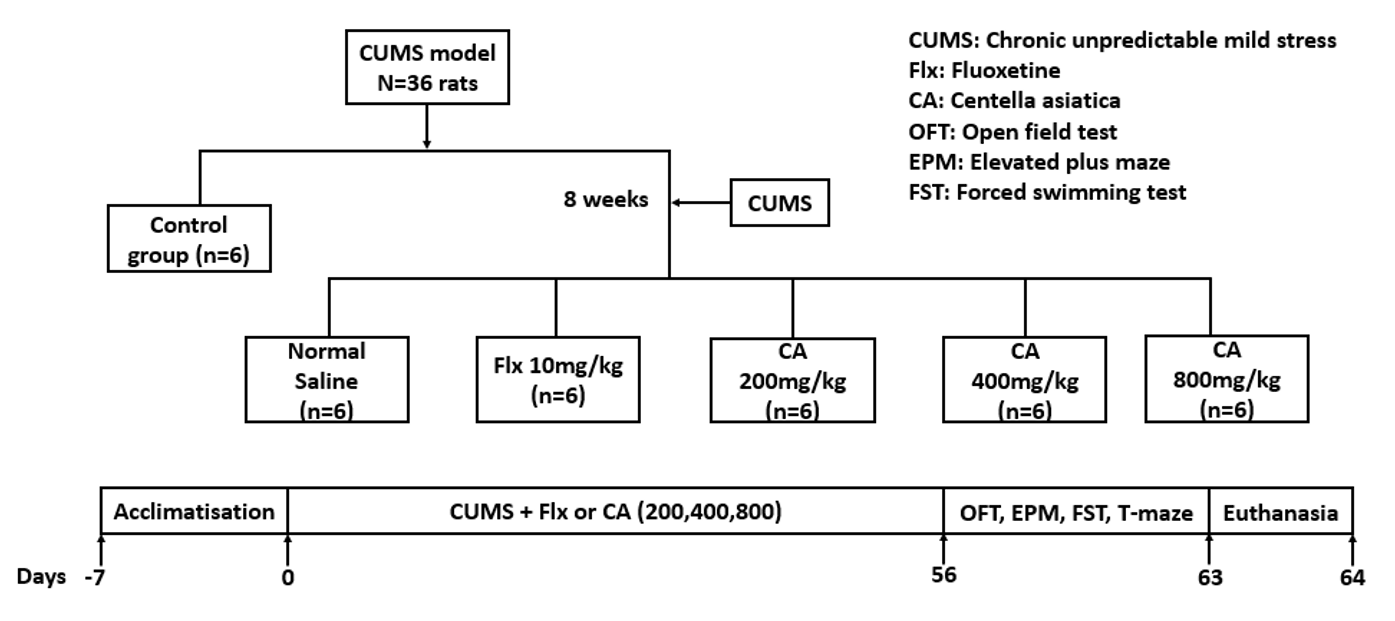
Experimental procedure for CUMS induction, treatments and behavioral tests, as well as abbreviations.
Open field test
The locomotor activity in rats was assessed through OFT, as described earlier 38,37. The device consisted of a square-shaped Plexiglas box with open top with a dimension of 75 cm in length x 75 cm in width (as the base) and 40 cm in height. The inner surface of the base was subdivided by lines into 25 smaller square units (15 cm x 15 cm), and is called the open field arena. A day before the test, rats were exposed to the device for 5 minutes to explore their novel environment for acclimatization. On the day of the test, each of the rats was placed separately at the centre of the open field arena and allowed to explore for 5 min. The total distance covered by each rat and the number of lines crossed was recorded by a video tracking system for later use. A rat was considered to have crossed the line if all of its four paws crosses the line. The device was cleaned with 70% ethanol in water and allowed to dry between tests to avoid olfactory cues.
Elevated plus maze
The EPM test was used to assess the level of anxiety in rats 39, using their natural tendency of rats to explore open spaces 40. The EPM device used for this study was previously described 24. The rats were individually placed in the center of the device, and their exploratory behaviors were recorded for 5 min. The parameters studied included the total number of entries by the rat into the open and closed arms of the device, and the time they spent at each visit to either the open arms or closed arms of the device. The time spent by the rat at the center of the device was not counted. After each trial, the maze was cleaned thoroughly with 70% ethanol and allowed to dry to eliminate any odor cues.
Forced swimming test
The FST was conducted based on the original method described by Slattery 41 with slight modification. Briefly, one day prior to the test, rats were individually subjected to a pre-test session in a transparent cylinder (20 cm wide and 50 cm high) filled with water (25 °C) at a depth of 30 cm, and left for 15 minutes for acclimatization. On the day of the test, the same procedure was repeated for 5 minutes and recorded with a video camera for later analysis. The duration of immobility was measured during the 5-minute test session. Immobility was defined as the absence of all movements, except the minor movements by the hind limbs of the rat to keep its head above the surface of the water.
T-maze spontaneous alternation
This test was conducted using a T-maze device fabricated from a dark plexiglass. The device was comprised of a long start arm (16 cm wide x 50 cm long) and two choice arms (10 cm wide x 10 cm long each) located perpendicularly at the top of the start arm. At the top (of that arm), a central dividing partition extended 15 cm into the start arm, forcing the rat to choose the left or right goal arm before reaching the convergence point 42. Rats were placed individually into the start arm of the maze, facing away from the convergence point and allowed to traverse the maze and choose a direction between the two goal arms. When the rat alternated the goal arms either left or right, the correction was recorded, and when it failed, the mistake was recorded. The choice was recorded only when the four paws had entered the arm. Each rat was given 7 trials separated by 30 s, for a possible total of 6 alternations between left and right arm choices.
Measurements of biochemical parameters
The rats were euthanized on the 64 day of the experiments, and brain samples were collected and kept at -80 ºC for later use. Blood samples were also collected in a plain bottle, centrifuged at 5000 x g for 5 minutes, and the serum was collected in clean eppendorf tubes.
Protein concentration
The total protein concentration of rat serum was measured using the bicinchoninic acid assay (BCA assay). Bovine serum albumin (BSA) (1 mg/ml) was used as a standard in the range of 0.01 – 0.1 mg/ml, for comparison with the total protein.
Enzyme-linked immunosorbent assay (ELISA)
The level of cortisol from rat serum was analyzed using the quantitative sandwich ELISA technique according to the manufacturer’s manual (Elabscience, USA). The optical density (OD) was then measured spectrophotometrically using a Versamax microplate reader (Molecular Devices, LLC, USA) at 450 ± 2 nm wavelength. The OD values proportional to the concentrations of cortisol were calculated from the standard calibration curves generated.
Statistical analysis
Using GraphPad Prism version 6 (ISI, San Diego, CA, USA) software, the data obtained were analyzed through one way ANOVA. Tukey's post hoc comparison was used where applicable; p < 0.05 were considered significant and results were presented as mean ± SD.
Results
Administration of CA attenuates CUMS-induced locomotor deficits in rats
The OFT was used to test the locomotor activity of the rats. One way ANOVA revealed statistically significant differences in their locomotor activity [F (5, 30) = 10.42, p = 0.0001] (Figure 2). The Tukey’s comparison showed statistically significant decrease in the number of lines crossed by CUMS-induced rats (39.3 ± 3.67, p = 0.006), when compared to control (51.1 ± 5.34). On the other hand, statistically significant increases in number of lines crossed were observed in CUMS-induced rats co-administered with Flx (54.67 ± 3.88, p = 0.0001), CA 400 (48.33 ± 7.50, p = 0.023) and CA 800 (50.67 ± 2.73, p = 0.0025), when compared to CUMS alone (39.33 ± 3.67). No statistically significant differences were observed among the control, Flx, and CA 400 and CA 800 groups of rats.

Effects of CA on locomotor activities in CUMS-induced rats as determined by OFT. Data presented as mean ± SD, n = 6. *p < 0.05
CA ameliorates anxiety-like behavior in CUMS-induced rats on EPM
Rats were tested for anxiety-like behavior through EPM and the one-way ANOVA analysis revealed statistically significant differences of time spent by rats on open arms [F(5,30) = 13.47, p = 0.0001]. Tukey’s comparison confirmed statistically significant decreases of time spent on the open arms by CUMS-induced rats (71.67 ± 11.5, p = 0.005) when compared to the control group of rats (162 ± 33.3). Significant increases of time spent on the open arm was also observed in CUMS-induced rats co-administered with Flx (211.8 ± 47.9, 0.0001), and CA at 400 mg/kg dose (149.9 ± 63.85, p = 0.02) and 800 (212.2 ± 42.8, p = 0.0001), when compared to CUMS alone (71.67 ± 11.5) (Figure 3A). On the other hand, no statistically significant differences were observed in the number of entries for the open arms by all groups of rats, as shown by one way ANOVA [F(3,20) = 13.47, p = 0.42] (Figure 3B).
Furthemore, one way ANOVA has revealed statistically significant differences of time spent by the rats on the closed arms [F(3,30) = 11.98, p = 0.0001]. Tukey’s comparison confirmed statistically significant increases of time spent in the closed arms by CUMS-induced rats (172 ± 8.71, p = 0.0018), when compared to the control group of rats (94.5 ± 10.60).
While significant decreases of time spent on the closed arms were observed in CUMS-induced rats co-administered with Flx (63.33 ± 29.5, p = 0.0001), CA 400 mg/kg (95.17 ± 48.55, p = 0.002), and CA 800 mg/kg (64.83 ± 31.69, p = 0.0001), when compared to CUMS alone (172 ± 8.71) (Figure 3C). Additionally, statistically significant increases of time spent in the closed arms were also observed in CA 200 mg/kg group (142 ± 34.92, p = 0.001) when compared to the Flx (63.33 ± 29.59). However, no statistically significant differences were observed between Flx, CA 400, and CA 800 groups of rats. Finally, one way ANOVA revealed no statistically significant differences in the number of times the rats entered into the closed arms of the maze [F(3,20) = 0.265, p = 0.849] (Figure 3D).
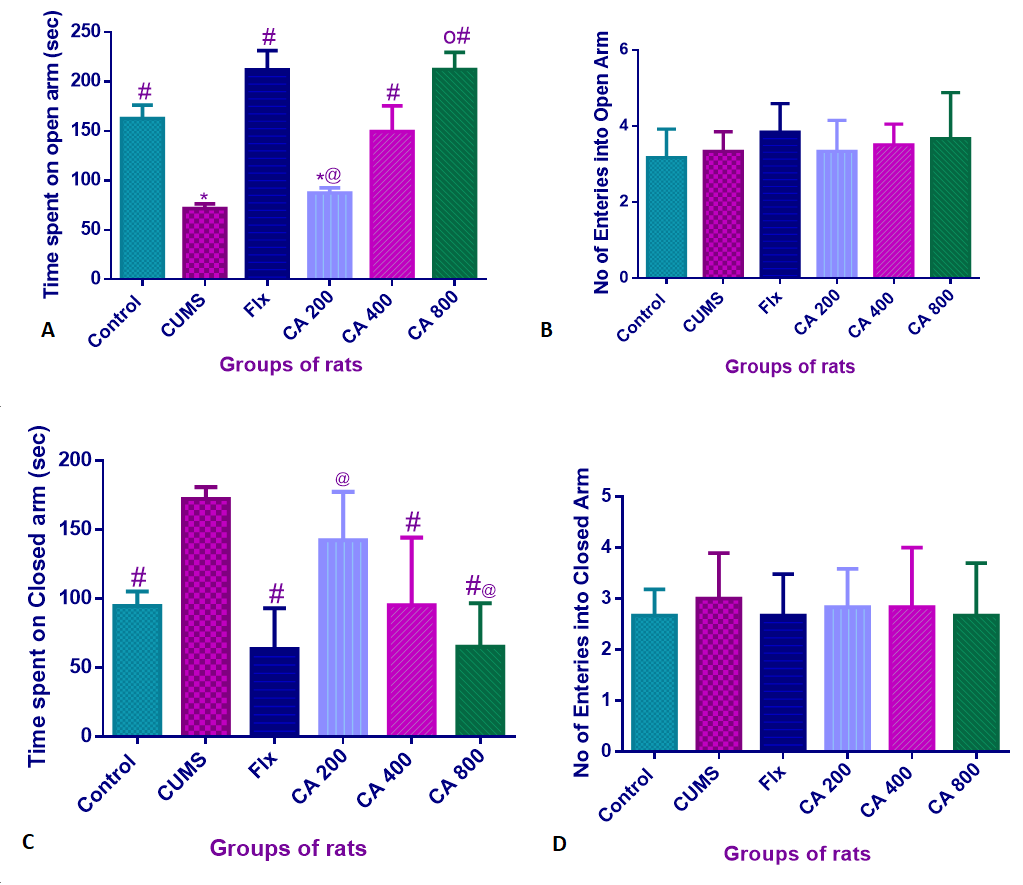
Anxiolytic effects of CA on CUMS-induced rats as determined by EPM. A. Time spent on open arms; B. Number of entries into open arms; C. Time spent on closed arms; and D. Number of entries into closed arms. Data are presented as mean ± SD, n = 6. *p < 0.05
CA prevents depression-like behavior in CUMS-induced rats as determined by FST
The protective effects of CA on depression-like behaviors in the rats were assessed via FST. One-way ANOVA showed statistically significant differences in the immobility time among the various rats groups [F(5,30) = 44.43, p = 0.0001] (Figure 4). Statistically significant increases in the immobility time were observed in CUMS-induced rats (10 ± 10.83, p = 0.0001), when compared to the control group (5 ± 1.4), as revealed by Tukey’s post hoc test. Whereas, statistically significant decreases of immobility time were observed in CUMS-induced rats co-administered with Flx (3.5 ± 1.37, p = 0.0001), CA 400(3.5 ± 1.64, p = 0.0001) and CA 800 (3.3 ± 1.2, p = 0.0001), when compared to CUMS alone (10 ± 10.83). No differences were observed between the control, Flx, CA 400, and CA 800 groups of rats.

Antidepressants effects of CA on CUMS-induced rats as determined by FST. Data presented as mean ± SD, n = 6. *p < 0.05
CA attenuates learning and memory deficits in CUMS-induced rats as determined by T-maze spontaneous alternation test
The effects of CA on learning and memory abilities of the rats were assessed through T-maze spontaneous alternation test. One way ANOVA revealed statistically significant differences in the number of correct alternations among the groups of rats [F(5,30) = 17.76, p = 0.0001] (Figure 5). Tukey’s post hoc revealed significant decreases in the number of correct alternations in CUMS-induced rats (50 ± 10.95, p = 0.0001) when compared to the control group of rats (91.6 ± 7.5). On the other hand, significant increases in the number of correct alternations were observed in CUMS-induced rats co-administered with Flx (88.3 ± 7.5, p = 0.0001), CA 400 (77.50 ± 8.8, p = 0.003) and CA 800 (85 ± 5.47, p = 0.0001), when compared to CUMS-induced rats alone (50 ± 10.95). No significant differences were observed among control, Flx, CA 400 and CA 800 groups of rats.
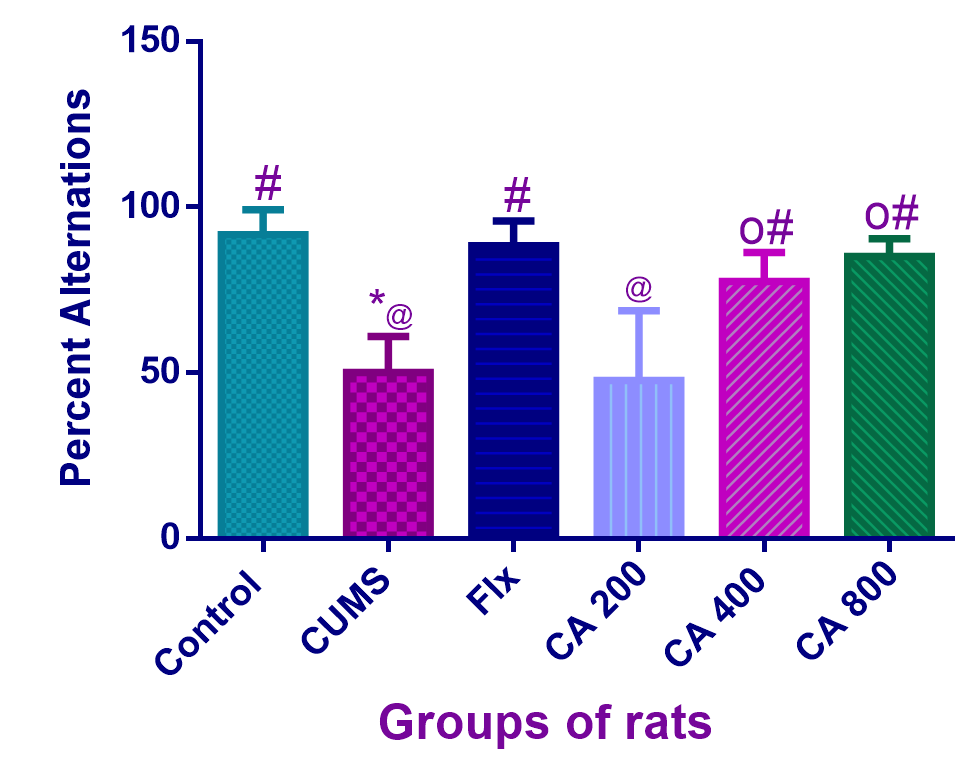
CA enhanced learning and memory in CUMS-induced rats as shown by T-maze test. Data presented as mean ± SD, n = 6. *p < 0.05
CA attenuates the levels of serum cortisol in CUMS-induced rats as determined by ELISA
The serum cortisol was evaluated in order to assess the therapeutic potentials of CA on CUMS-induced rats. One way ANOVA showed statistically significant differences in the level of serum cortisol among the groups of rats [F (5, 12) = 2.264, p = 0.0001] (Figure 6). The Tukey’s post hoc comparison revealed significant increases of serum cortisol levels in CUMS (36.15 ± 1.045, p = 0.0001) and CA 200 (34.64 ± 0.72, p = 0.0001) groups of rats, when compared to control (20.10 ± 0.56). On the other hand, statistically significant decreases in the level of serum cortisol were observed CUMS-induced rats co-administered with Flx (22.08 ± 0.507, p = 0.0001), CA 400 (22.07 ± 0.581, p = 0.0001) and CA 800 (21.30 ± 1.298. p = 0.0001), when compared to CUMS-induced alone (36.16 ± 1.045). No statistically significant differences were seen with the Flx, control and CA (400 and 800) group of rats. Although, statistically significant different decrease of serum cortisol level was seen in CA 400 (22.07 ± 0.581, p = 0.0001) and CA 800 (21.30 ± 1.298. p = 0.0001), when compared to CA 200 (34.64 ± 0.72).

CA attenuates serum cortisol level in CUMS-induced rats as shown by T-maze test. Data presented as mean ± SD, n = 6. *p < 0.05
Discussion
In the present study, rats subjected to CUMS were used as a model of depression to compare the effects of fluoxetine (an established antidepressant) with those of CA, which has not been previously tested in a CUMS model of depression. Major findings from this study have shown that CUMS resulted in anxiety and depression-like behaviors, as well as learning and memory impairments in rats. These behavioral changes were also parallel with the high level of serum cortisol, which is suggestive of stress induction. Administration of CA, efficiently ameliorated the anxiety, depression-like, and cognitive deficits associated with CUMS exposure.
Additionally, decreases in the level of serum cortisol were observed in the fluoxetine and CA (400 & 800 mg/kg) groups of rats. Thus, fluoxetine and CA follow similar stress ameliorating pathways. These findings strongly suggest the anxiolytic, anti-depressant, and anti-amnesic effects of CA in CUMS-induced rat models. Based on the existing literature searched, this is the first report on the therapeutic properties of CA in CUMS-induced rats.
CUMS is being used widely for studying antidepressants because of its reliability 36,43 and its link to many neuropsychiatric disorders, such as depression and anxiety44. The CUMS is one of the well-established protocol for inducing depression-like behaviors in rats because it could induce stress in an unpredictable manner 45,46 and can be likened to the unpredictable stressors of human life with good predictive, face and construct validity 47. Therefore, a CUMS model was used to evaluate the anxiolytic, antidepressant-like, and cognitive enhancing effects of CA in this study. Nevertheless, the influence of CA on depression-like behaviors has been examined earlier in other animal models 26,27,48.
OFT is often used to quantitatively and qualitatively measure the general locomotor activities and willingness of rodents to explore their novel environments 49. Therefore, OFT and actophotometers used in developing animal models assesses rodent’s exploratory and locomotor behaviors 35. In the present study, rats exposed to 8 weeks of CUMS had reduced locomotor activities, as demonstrated by a significant reduction of number of lines crossings in the OFT suggesting anxiety-like effect. A similar result was reported 37, where the rats exposed to CUMS covered total less distance, reduced rearing and less line crossing suggesting a change in security and emotion. Treating CUMS-induced rats with Flx 10 mg/kg or CA (400 and 800 mg/kg) reversed the locomotor deficits, which is evident by the increased number of lines crossed. Previous studies have shown that treatment with fluoxetine a selective serotonin re-uptake inhibitor reversed depression-related behaviors in CUMS-induced rats35,50, which is consistent with the current work. While CA at the doses of 400 and 800 mg/kg have similar effects like fluoxetine at 10 mg/kg, suggesting anxiolytic effects of CA on CUMS-induced rats.
EPM evokes conflict between the need to explore the novel area and the rodent's natural reluctance to avert vulnerable areas of the EPM (open spaces and heights) 26. Hence, EPM test was used to assess the degree of anxiety in rodent 39. Enhancement of anxiety in response to CUMS exposure has been earlier reported by investigators who studied the effects of various antidepressants on anxiety 37,51. In accordance with previous reports, rats exposed to CUMS showed enhanced anxiety and they spent a shorter time on open arms and longer time on the closed arms of the EPM when compared to control group of rats. Co-administration of fluoxetine (10mg/kg) to CUMS-induced rats reversed the aforesaid anxiety-like behaviors. As seen in fluoxetine group of rats, administration of CA at doses 400 and 800mg/kg during CUMS exposure also produced anxiolytic effects which are evident from the in EPM test. In agreement with this study, a significant anxiolytic activity of ECa 233, a standardised extract of CA was reported in mice subjected to chronic immobilised stress. As the mice showed an increased frequency of visit to the open arms and spent longer time exploring the open arms 27. Similarly, asiaticoside an active phytochemical isolated from CA was reported to have anxiolytic effects in a different behavioral rat model of depression 26.
The FST is a method widely used for the assessment of depressive-like behaviors and the evaluation of the efficacy of anti-depressive drugs in rodents 52,53. It is based on the tenet that animals in an enclosed environment become immobile after an initial period of strenuous activity and that the duration of immobility decreases by effective anti-depressants agents 53. As accordance with results reported from earlier studies 36,37, the rats exposed to CUMS in this study also presented with depression-like behaviors as they spent longer period of immobility in the water tank when compared to the control rats. Conversely, CUMS-induced rats co-administered with fluoxetine (10 mg/kg) or CA (400 and 800 mg/kg) do not exhibit depression-like behaviors since they showed longer mobility time in the water. Anti-depressant effects of CA and its phytochemical compound asiaticoside, were reported from different rodent models of depression 27,48,54, which are in line with the present work. Since no marked differences were observed in depressive-like behaviors between fluoxetine (10 mg/kg) and CA (400 & 800 mg/kg) treated groups of rats, it can be suggested that CA has antidepressant-like properties similar to that of fluoxetine.
The mood disorders in depressive patients are often associated with cognitive symptoms, such as difficulty in decision-making, deficits in learning and memory, and loss of cognitive flexibility 3. The T-maze test could be used to assess cognitive functions in rats 42, as an alternation. Whether rewarded or spontaneous the T maze test is an excellent method for identifying hippocampal dysfunction possibly even better than Morris water maze test55. Rodents exposed to CUMS exhibit cognitive dysfunctions, as confirmed by some reports of the earlier studies 5,36, and the results from the present study in accordance with the previous reports. As CUMS-induced rats showed higher number of wrong alternations when compared to the control rats who scored higher number of correct alternations. However, CUMS-induced rats co-administered with fluoxetine (at 10 mg/kg) or with CA at doses 400 or 800 mg/kg reversed the cognitive deficits that were observed in CUMS alone induced rats group. Since no significant differences were observed between fluoxetine and CA (400 & 800) groups of rats concerning their cognitive behaviors, it can be safely concluded that CA at these doses has similar effects to fluoxetine in attenuating stress-induced cognitive deficits. Different authors have reported neuroprotective effects of CA on numerous neurodegenerative disease models and in the settings of different pathological insults 12,56,57,58.
The mechanistic pathways involved in depression and the actions of antidepressants are complex and multifactorial. As chronic stress can lead to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which is accompanied by the disproportionate generation of glucocorticoids leading to impaired hippocampal function and depression-like behaviors 59,60. Antidepressants like imipramine and fluoxetine have been shown to attenuate these behavioral changes61. Elevated concentrations of cortisol have been reported in patients with depression, however, antidepressants such as fluoxetine have shown to reduce the elevated cortisol concentration 62. In the present study, higher concentrations of serum cortisol were observed in rats exposed to CUMS, and these were considerably reduced by fluoxetine and CA (400 & 800). In this study, fluoxetine (a selective serotonin reuptake inhibitor (SSRI) was used) as a positive control. CA produced similar effects to fluoxetine, although the effects of fluoxetine are slightly higher than those of CA in most of the parameters measured. This research, therefore, speculates, that CA might work in a similar fashion to fluoxetine. Future follow-up studies of fluoxetine and CA will need to be conducted for further verification. This study was limited to behaviors and serum cortisol levels (when exploring the effects of CA on CUMS-exposed rats). Various other brain regions, such as the hippocampus, olfactory bulb and prefrontal cortex, are also affected by CUMS and should be explored63.
Conclusion
In conclusion, results from the present study showed that CA could prevent CUMS–induced behavioral changes in rats as well as reduce the concentration of cortisol. These effects of CA (at doses of 400 & 800 mg/kg) is similar to that of fluoxetine, although fluoxetine is better. This study suggests that CA might be a novel neuroprotective nutraceutical and phytochemical agent in future neurodegenerative and depression research. Molecular studies are ongoing to elucidate the probable mechanisms through which CA exerts its effects on CUMS-induced rats.
Abbreviations
ANOVA: Analysis of variance
BDNF: Brain-derived neurotrophic factor
CA3: Connus amonis 3
CA: Centella asiatica
Cm: Centimetre
CREB: cAMP-response element binding protein
CUMS: Chronic unpredictable mild stress
°C: Degree celcius
EPM: Elevated plus maze
FLX: Fluoxetine
FST: Forced swimming test
h: Hour
HPA: Hypothalamic-pituitary-adrenal
Kg: Kilogram
Mg: Milligram
Min: Minutes
OFT: Open field test
S: Stressor
SD: Standard deviation
SSRI: Selective serotonin reuptake inhibitor
USA: United States of America
Competing Interests
The authors report no conflicts of interest in this work.
Authors' Contributions
SJ, MAMM, CNMT, MIA, ZA and MTHB conceptualized and designed the work. SJ and SMC carried out the experiments and write the drafts. All authors approved the manuscript.

