Abstract
Today, the late diagnosis of cancers is a big challenge, and using novel diagnostic techniques will provide essential, faster and more accurate treatments. Unfortunately, existing common and traditional diagnostic methods have not been helpful completely and most cancers are diagnosed too late. Recently, researchers have found new diagnostic methods against cancers by aptasensors; these sensory systems can detect involved biomarkers in various cancers so that the research in this field is continued strictly. Aptasensors can detect cancer markers in small quantities and high selectivity; moreover, other advantages of cancer aptasensors such as optimized time and cost saving can be considered. In addition, the aptasensors have been used in the diagnosis of the effective and related factors in cancer therapy follow-up. Here, the most researches about cancer aptasensors and other involved markers were collected, reviewed and described.
Introduction
Changes in the traditions of lifestyle have led to the transform the dietary patterns, including increased intake saturated fat and reduced the fruits and vegetables, air pollutant, stress, different waves, etc. These major elements have led to the increased prevalence of non-communicable diseases such as cardiovascular disease, cancer, diabetes, etc1,2,3,4,5,6. The sudden and uncontrolled growth of cells in a part of the body caused by genetics and inheritance, some viruses, environmental hazards (smoking, ionizing radiation, and chemicals) or pattern and lifestyle in terms of diet, the reduced time of exercise and increased stress are considered as the related risk factors of various cancers7,8,9,10,11. Annually, millions of cancer cases are found in the world. At the early stages, the diagnosis of cancerous cells are limited and complicated12,13,14,15; One of the main problems in the cancer treatment is the lack of a suitable method for early diagnosis16,17. The big challenge associated with various cancers is the delayed diagnosis and the occurrence of metastasis18,19. However, the detection of cancers at the early stages is usually impossible with existing methods20,21. Delay in the treatment will lead to metastasis20,21. Along with the late diagnosis, cancer involves other organs and tissues leading to difficult treatment process20,21. One of the main goals of the researches is finding biomarkers to detect cancer at early stages22,23,24. Generally, biomarkers refer to a group of measurable indicators of some biological conditions25,26. Biomarkers are often evaluated for the study of natural biological processes, the diagnosis of pathogenic processes or the response to a specific treatment. In recent years, special biomarkers have been used extensively to identify various diseases. Non-invasive, sensitive and cost-effective measurement, stability in the samples, specified diagnosis, early identification before the onset of symptoms, are main characteristics that can be desirable for a biomarker27,28. Over the past three decades, the use of synthetic DNA or RNA single-stranded oligonucleotides (aptamers) was expanded in various fields of biology and medicine in through different ways29,30,31. Aptamers can capture their molecular targets with high affinity and specificity. These properties are related to the ability of this category of biological molecules to interact with other molecules by refolding and conformational changes32,33. Aptasensors are kinds of biosensors that aptamers (DNA or RNA oligonucleotide sequences in a single strand status) act as the biorecognition element in their structure34,35. Given that the used aptamers in aptasensors are synthetic, the affinity and specificity of aptasensors towards the analytes can be controlled and enhanced36,37. Aptasensors can detect analytes in very small amounts where these minimalistic amounts are not detectable with most of the other existing methods38,39; in addition, the low cost for designing the aptasensors compared to other cancer diagnostics methods should be considered as one of the economic savings40,41. Other considerable advantages of aptasensors compared to other available diagnostic techniques are low detection time and fast detection process 34,36,39,40,42. In this review, the applications of aptasensors in order to the diagnosis of cancer biomarkers were collected and reviewed from related researches.
Electrochemical aptasensors
Electrochemical biosensors are attended by numerous advantages, including simplicity, low cost, high selectivity and high sensitivity. Hence, these sensors have become powerful tools in various biomedical fields, especially the diagnosis of diseases. Electrochemical sensors are typically classified as amperometric sensors, potentiometric sensors, and impedance sensors. Electrochemical sensors consist of three main components, including biorecognition element, transducer and detector. In electrochemical aptasensors, the biorecognition element is aptamer. This biorecognition element is usually chosen to have the most affinity against analyte. The principle of diagnosis in electrochemical aptasensors is based on redox behavior. In the absence of an analyte in the environment, the redox molecules are placed at a specified distance near the aptamer, which will lead to recording an electrochemical current. In the presence of analyte molecules in the environment, due to the specific affinity between aptamer and analyte, analyte molecules are captured and absorbed by aptamer molecules, which lead to the placement of redox molecules at different distances; this approach creates and records a different electrochemical current 43,44,45,46. By evaluating and comparing recorded electrochemical currents, it is possible to detect analytes quantitatively and in very small amounts by these electrochemical aptasensors.
Aptasensors for detection of vascular endothelial growth factor (VEGF)
In a research, Qureshi et al. tried to design an aptasensor in order to detect the VEGF in human serum47. VEGF as a signaling angiogenesis protein is related to some cancers; this research tried to use the dielectric measurements between aptamer and surface of the gold electrode in the absent/presence of VEGF. They offered an early and sensitive diagnosis method for VEGF in the range of 0.5 to 2 ng mL-1. In another research, designing an electrochemical aptasensor for detection the human mucin-1 (MUC1) and VEGF tumor markers were investigated by Zhao et al. 48. They used of a three-electrode system whereas the working electrode was gold and the redox marker was ferro/ferricyanide [Fe(CN)6 3−/4−]. The results of this study showed that this aptasensor was able to detect MUC1 and VEGF tumor markers up to 20 nM (as the highest response). Cho et al. designed an aptasensor for early detection of VEGF165 49. The detection range of this experimental work was in a linear range from 25 pg mL-1 to 25 μg mL-1. In this work, the gold nanoparticles were used in order to improve the metal fluorophore interactions; the authors compared all achieved experimental results with enzyme-linked immunosorbent assay (ELISA) and this comparison confirmed the successful design of VEGF165 aptasensor. Amouzadeh Tabrizi et al. tried to design an electrochemical aptasensor in order to detect of VEGF165 tumor marker (10.0 to 300.0 pg mL-1) in the serum of lung cancer patients50. In their electrochemical measurements, several screen-printed electrodes (SPE) were used and main applied electrochemical detection techniques were cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) in an electrolyte containing [Fe(CN)6 3−/4−] as redox marker. Another aptasensor was designed to detection the VEGF (0 to 200 nM) based on surface plasmon resonance (SPR)51.
Aptasensors for detection the prostate-specific antigen (PSA)
PSA is considered as the major prostate cancer marker within the blood samples of patients; In a research Jolly et al. tried to design an aptasensor for PSA 52; their analyses were determined based on the EIS method. The low detection ability of the analytes is a very important aim in all aptasensors and diagnostic methods; The PSA was detected in the range lower than 1 ng mL−1 in the mentioned work. In another research, the PSA detection was followed through a nanoaptasensor where the gold nanoparticles were encapsulated by graphitized mesoporous carbon 53. In this research, the electrochemical detection technique was differential pulse voltammetric (DPV) and this aptasensor could detect PSA in the patient’s serum in the range of 0.25 to 200 ng mL−1. Designing an electrochemical aptasensor against PSA antigen using goldnanospears was followed by Rahi et al. 54. They tried to detect PSA in the range of 0.125 to 200 ng mL-1 and the limit of detection (LOD) in their research was about 50 pg mL-1. In the mentioned research, a 32 mer thiolated aptamer (5’-SH) was used in order to find optimized binding surface against the analyte. In their work, the methylene blue (MB) was used as the redox marker and all electrochemical measurements were based on CV and DPV techniques. Designing an electrochemical PSA aptasensor was followed using the carboxylic acid functionalized by carbon nanotubes (CNTs(COOH)), chitosan (Chit), carbon nanotubes-chitosan (CNTs-Chit and CNTs(COOH)-Chit) 55. Here, all electrochemical studies were performed through CV, DPV, and EIS while the LOD for this aptasensor was 0.75 ng mL-1. Another PSA aptasensor designing was followed based on chemiluminescence 56; in this research, the PSA was detected in the range of 1.9 to 125 ng mL−1.
Aptasensors for detection the breast cancer MCF-7 cells
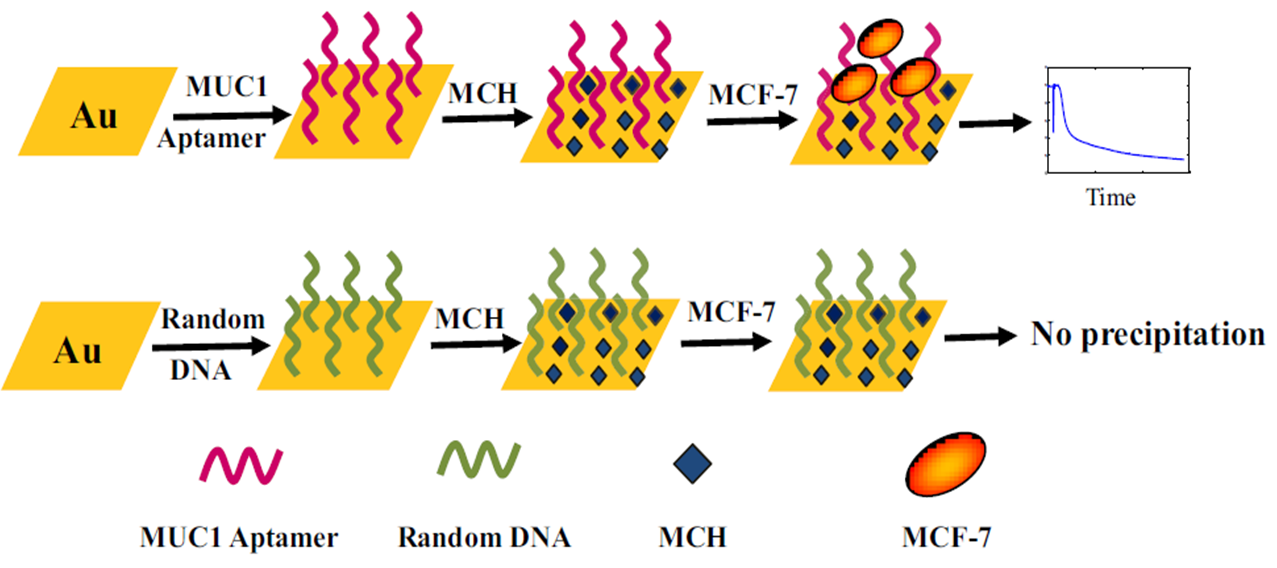
In a research, the breast cancer diagnosis was followed by Cai et al. 57; they designed an aptasensor that was able to detect breast cancer MCF-7 cells in the range of 0 to 500 cells mL-1. Electrochemical results were obtained via chronocoulometry (CC), EIS and CV while the redox marker was [Fe(CN)6 3−/4−]. In another research, Chang et al. offered an aptasensor in order to detect of the MCF-7 cells 58. The detection plan was based on the leaky surface acoustic wave (LSAW- 100 MHz) aptasensor array. This aptasensor could detect the MCF-7 cells in the range of 1×102 cells mL-1 to 1×107 cells mL-1; while the LOD was about 32 cells mL-1. Choosing a specific aptamer against analytes is very important in all aptasensors 59. In the mentioned research, the authors used of a specific MUC1 aptamer in order to detect of the MCF-7 cells; in Figure 1, by using the specific aptamer, the mentioned aptasensor was able to detect the MCF-7 cells while when the random DNA was used, the aptasensor did not work properly.
In a research, a fluorescent aptasensor was designed to detect the MCF-7 cells 60. Cai et al. used fluorescence analysis as the main detection method and this aptasensor was able to detect the MCF-7 cells in the range of 500 to 5.0×105 cells mL-1 while LOD was 70 cells mL-1. In another research, a graphene oxide (GO) based aptasensor was designed that could detect the MCF-7 cells (180 to 8×107 cells mL-1), HL-60 (210 to 7×107 cells mL-1), and K562 (200 to 7×107 cells mL-1) cancer cells61. In this aptasensor, the mesoporous silica nanoparticles (MSNs) were used and all analysis were followed through visual fluorescence measurements. Li et al. introduced an aptasensor to detect the MCF-7 cells while the gold nanorods were used as a signal amplifier; the detection range was 102 to 105 cells mL-1 and LOD was calculated as 100 cells mL-1 62. The MUC1 aptamer-functionalized gold nanorods applied in mentioned aptasensor and the all obtained results were analyzed through the localized surface plasmon resonance (LSPR) spectra. A colorimetric nanoaptasensor was designed using the aggregation of gold nanoparticles; after investigation of the absorption spectra, it applied to detect the MCF-7 cells in the range of 10 to 105 cells 63. In a research, the MCF-7 cells were determined (100 to 5.0 × 107cells mL−1) via an electrochemical aptasensor that used of the GO/Au composites and porous PtFe alloy as the signal amplifier 64. The LOD for this aptasensor was reported as 38 cell mL−1. A fluorescent aptasensor was introduced by Li et al. that was based on the mesoporous carbon nanospheres 65. This aptasensor presented a special way in order to detect the of MUC1 tumor marker molecules (0.110.6 μmol L−1) and MCF-7 cells (104-2×106 cells mL−1). A photoelectrochemical aptasensor was designed to detect the MCF-7 cells 66. This aptasensor could detect the MCF-7 cells at the range of 1×103 to 1×105 cells mL−1.
Aptasensors for detection the Ramos cells
An electrochemiluminescence aptasensor was developed to detect the Ramos cells (CRL-1596, B-cell, human Burkitt's lymphoma) via application of the Au and silica nanoparticles and magnetic beads67. This aptasensor could detect the Ramos cells in the linear range from 100 to 2000 cells mL−1. A nanoaptasensor (aptamer-nanoparticle strip biosensor) was designed by Liu et al. due to detection of the Ramos cells in the human blood samples 68. The LOD for this aptasensor was about 800 Ramos cells.
Aptasensors for detection the tumor necrosis factor-α (TNF-α)
TNF-α is an anticancer agent used in cancer therapy through isolated limb perfusion and prevention amputation of the limb 69. Experimental design of an aptasensor in order to detect the TNF-α was investigated by Liu et al. 70. This research is one of the related tries to detect the biomaterials that have a role in cancer therapy. The performance mechanism of this aptasensor is shown in Figure 2.
In this research, the MB was used as redox marker in all electrochemical analyses. The MB molecules were attached to 5’end of RNA aptamer. All electrochemical measurements were performed through square wave voltammetry (SWV) and the LOD was 10 ng mL-1 for TNF-α. another research showed a try in designing an aptasensor for detecting the TNF-α at the range of 0.001 ng mL-1 to 100 ng mL-171. Here, the cucurbituril 7 (CB)/nano gold@chitosan was used as the signal facilitator and this led to finding the LOD about 0.5 pg mL-1. In addition, as mentioned in section 3, TNF-α detection was also applied for prostate cancer diagnosis 70.
Aptasensors for detection the leukemia cells
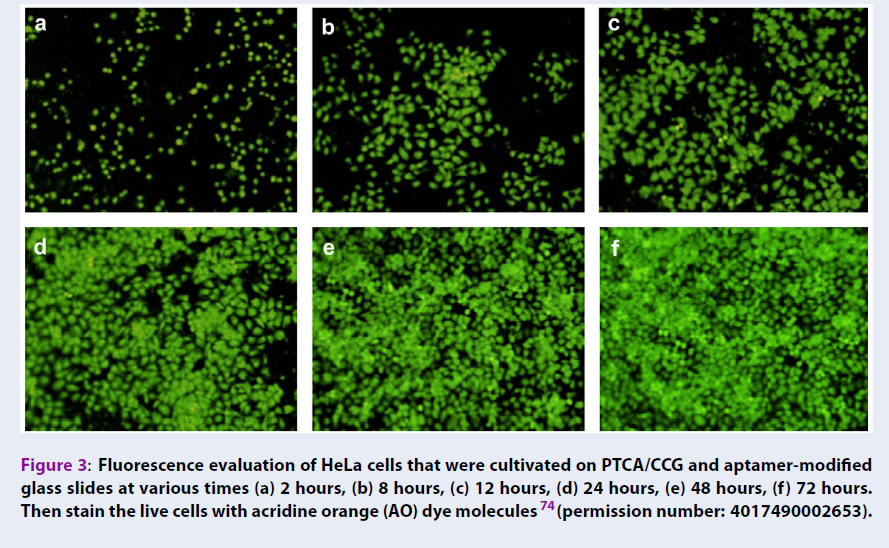
In a research, Cao et al. tried to investigate a signal-on aptasensor in order to detect the T-cell acute lymphoblastic leukemia cells (CCRF-CEM cells)72; they used of GO-based fluorescence resonance energy transfer (FRET) in the sensing strategy and the detection results showed a linear range (2.5 × 101 to 2.5 × 104 cells mL-1) against CCRF-CEM cells. In another research, in order to detect of the adenosine in the cancer cells, an electrochemiluminescence aptasensor using a wireless indium tin oxide bipolar electrode (BPE) was reported 73. This aptasensor could detect the adenosine triphosphate (ATP) in a linear range from 1.0 fM to 0.10 μM. The results of this research were used in the diagnosis of K562 leukemia cells. Graphene was used in an electrochemical aptasensor for detection of the HeLa cells (human cervical carcinoma cell), MDA-MB-231 (human breast cancer cell), K562 cells (leukemia line), and NIH3T3 cells (mouse embryonic fibroblast cell line) 74. The growth and proliferation of HeLa cells and their viability at various times on 3,4,9,10-perylene tetracarboxylic acid PTCA-functionalized chemical converted graphene (PTCA/CCG) are shown in Figure 3.
The results of this research showed the more successful detection method for HeLa cells (human cervical carcinoma cell) and MDA-MB-231 (human breast cancer cell). In this research, electrochemical measurements were performed through CV and EIS and the redox marker was K3[Fe(CN)6]. An electrochemical aptasensor was designed to detect HeLa cells at the range of 10 to 106 cells mL−175. This aptasensor was based on the graphene nanocomposites that this material led to amplification of the detectable signal produced by the analyte. A visual colorimetric aptasensor was designed based on cell-triggered cyclic enzymatic signal amplification 76. This aptasensor could detect T-cell acute lymphoblastic leukemia cells (CCRFCEM cells) in the range of 102 to 104 cells and the AuNPs with a diameter of 13 nm were used as the aptamer modifier. Using an electrochemiluminescence adenosine aptasensor was followed by Tian et al. 77. Photocatalytic properties of TiO2 nanotubes were used in the mentioned aptasensor and it showed a 3.32 ± 0.2 pmol/cell detection value against K562 cells.
Aptasensors for detection of MUC1 cells and MUC1 application in them
Over-expressing the MUC1 glycoprotein is the very important sign and marker to detecting cancer; A fluorescent aptasensor was designed to detect the MUC1 78; the GO was used as the signal quencher. In this research, the analyses were performed through a fluorescence spectrophotometer. He et al. could detect MUC1 in the range of 0.04 to 10 μM. In another research, Liu et al. designed an aptasensor in order to detect of the MUC1 tumor marker 79; in this research, electrochemical measurements were performed through EIS technique and the redox marker was [Fe(CN)6 3−/4−]. Liu et al. applied two work methods due to detecting the MUC1 (Figure 4); they used of bare gold electrode and modified gold electrode with Au nanoparticles; when they used of the modified gold electrode with Au nanoparticles, the aptasensor showed a significant amplification response against MUC1 and this situation was related to three-dimensional structure and conformation changes in the used thiolated DNA aptamer when Au nanoparticles existed.
According to the author’s claims, this aptasensor was able to detect MUC1 at very low concentration (0.1 nM) and showed the high selectivity, reproducibility, and stability. Other related researches in order to detect of the MUC1 also was mentioned before in section 248. In an aptasensor, EIS and CV techniques were used to detect the colon cancer DLD-1 cells80. The authors of this research used of MUC1 aptamer and carbon nanospheres (CNSs) with an average diameter of 500 nm; using of the conjugated MUC1 aptamer with CNSs had led to the detection of the colon cancer DLD-1 cells in the range of 1.25×102 to 1.25 ×106 cells mL−1. Cao et al. tried to offer an early diagnosis method for diagnosis of the colon cancer through detection the MUC1 glycoprotein on the cellular membrane. As previously cited, Chang et al. used a specific MUC1 aptamer in order to diagnosis the MCF-7 cells 58. Other try for detection the MUC1was related to Li et al. research that was presented in section 4 65.
Aptasensors for detection the carcinoembryonic antigen (CEA)
An electrochemiluminescence aptasensor was designed to detect the CEA in the real human serums samples81; in this aptasensor, cadmium sulfide graphene nanocomposites were used as coreactant. This aptasensor could detect CEA at the range of 0.01 to 10.0 ng mL-1. In other work, electrochemiluminescence CEA assay was investigated through a nanoaptasensor containing nanocomposites (ZnS-CdS nanoparticles (NPs)-decorated molybdenum disulfide (MoS2). The mentioned nanomaterials were used to provide a high loading analyte capacity 82; this aptasensor showed a linear detection range (0.05 to 20 ng mL-1) versus CEA. A FRET based aptasensor was designed to detect CEA 83. In this aptasensor, carbon nanoparticles (CNPs) and an amino group-modified aptamer were used, and the found results showed a linear detection range (0.1 to 40 ng mL-1) against CEA. An electrochemical aptasensor was designed to detect the CEA. In the structure of this aptasensor, a nanocomposite of gold nanoparticles, hemin, and graphene were applied 84. This aptasensor could detect CEA in the range of 0.0001 to 10 ng mL-1 and LOD for it was 40 mL-1. In Table 1, most usual methods used for detection of CEA are cited.
| Detection technique | Detection range | Reference |
| Electrochemiluminescence aptasensing | 0.01–10.0 ng mL-1 | 81 |
| Electrochemiluminescence aptasensing | 0.05 to 20 ng mL-1 | 82 |
| FRET based aptasensor | 0.1 to 40 ng mL-1 | 83 |
| Electrochemical aptasensing | 0.0001 to 10 ng mL-1 | 84 |
| Qualitative Analysis | cutoff value was 2.5 ng/L | 90 |
| Qualitative Analysis | cutoff value was 7 mg/L | 85 |
| Qualitative Analysis | up to 32 μg/ L | 86 |
| Luminescence Assay | --------- | 87 |
| latex photometric immunoassay (LPIA) | --------- | 88 |
| Qualitative Analysis | 10 ng ml-1 | 89 |
Other applications of aptasensors in the cancer follow-up
In a research, an aptasensor was designed to detect the cytochrome c 91. This aptasensor was used to investigate the drug treatment in the liver cancer; the screen mechanism was related to diagnosis of cytochrome c; cytochrome c role in apoptosis as one of the fighter processes against cancer is clear, so that the B-cell lymphoma 2 (Bcl-2) regulatory protein is the cause in order to release the cytochrome c from the mitochondria, which leads to the activation of caspase-9 and then caspase-3; eventually, this pathway leads to cell apoptosis 92. A way analytics method to detect the cytochrome c was SPR that performed through common-path spectral interferometry. Loo et al found an aptasensor that could detect cytochrome c in the range of 80 nM to 80 pM. In another electroanalytical work, Kara et al designed an aptasensor for detecting the adenocarcinoma type lung cancer. In this research, the cytosensing platform and EIS technique with [Fe(CN)6 3−/4−] as redox marker were used 93. The obtained LOD for this aptasensor was 163.7 cells mL−1; the selectivity of this aptasensor was compared with other cancers (human hepatic cancer HepG2 & human cervical cancer HeLA cells); the final results of the mentioned work showed that this aptasensor was highly selective versus A549 cell line. Designing a specific aptasensor in order to detect the human hepatoma SMMC-7721 cells was followed by Yuan et al. 94. This aptasensor was based on FRET and the found results were approved with flow cytometry assay. In this study, the Cy3 and Cy5 fluorescent group were labeled and applied on the used aptamer; during detection of the analyte, the FRET signal appeared for low amounts of SMMC-7721 cells (LOD: 20 cells in 200 μL). An electrochemical aptasensor was designed to detect the LC-18 tumor marker in lung cancer patients 94. The LOD against LC-18 was about 0.023 ng mL-1. In electrochemical studies, SWV was used as the main analyte analysis technique. In a research, Hashemian et al designed a fluorescence aptasensor based on FRET (between CdS quantum dot (QDs)) in order to the detection of adenosine in urine samples of the lung cancer patients 90. This aptasensor could determine the adenosine in a linear range of 23 to 146 nM while LOD was 9.3 nM. Qureshi et al. tried to offer a new way for detection of the human epidermal growth factor receptor 2 (HER2) cancer biomarker (0.2 to 2 ng mL−1) 92; in this aptasensor, the detection method was based on changing the dielectric parameters (impedance/capacitance). Visual monitoring of cancer biomarker anterior gradient homolog 2 (AGR2) was followed through an aptasensor using UV–vis spectrometry 93. This aptasensor could detect AGR2 at the linear range of 10 to 1280 pM with LOD 6.6 pM. An aptasensor was introduced for liver cancer through the detection of HepG2 cells in the range of 1×103 to 1×105 cells mL−194. Analytical section of this aptasensor was based on commercial cantisens sensor platform and microcantilevers.
Conclusion
We know, regardless of nationality, cancers are one of the main mortality causes in the world. The exact cause of cancer is unknown and likelyprobable genetic factors or other issues that interfere with the activity of cells are involved in the incidence of these diseases. The aims of medicine are health promotion, maintain, restore health, reduce pain and reduce disability. One of the most important steps to achieve this goal is to prevent the spread of cancers when they are in the early stages. Moreover, trying to make ways for early diagnosis and also choosing the appropriate treatment are very necessary against cancers. The extensive researches are performed in order to find early detection, low cost, easy and accurate methods against cancers. The routine cancer diagnostic applied methods are often invasive and expensive; in addition, in very small quantities, these methods cannot detect early tumors. Aptamers as molecular structures have been used in the structure of aptasensors in order to diagnosis the many cancers with high affinity. Using advanced nanostructures and the functionalized aptamer with some organic materials has increased the diagnostic sensitivity and specificity of aptasensors. As the specificity and sensitivity of the aptasensors is been higher, makes them appropriate commercial diagnostic tools for the early diagnosis of cancers. It is hoped that the researches will reduce the effects of interfering factors in cancer aptasensors; also, by integrating other technologies, the more accurate and portable diagnostic cancer tools such as commercial aptasensors will be produced.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
All authors contributed substantially to the conception and design of the study.
Acknowledgments
We would like to thank the Research Council of Shiraz University of Medical Sciences (20336) for supporting this research.
References
-
Kunst
A.,
Stronks
K.,
Agyemang
C.,
Non-communicable diseases. Migration and health in the European Union.
2011;
1
(116)
.
-
McQueen
D.V.,
Global Handbook on Noncommunicable Diseases and Health PromotionSpringer: New York; 2016.
Google Scholar -
Shankar
S.,
Srivastava
R.K.,
Nutrition, Diet and CancerSpringer: Netherlands; 2015.
Google Scholar -
Stanner
S.,
Foundation
B.N.,
Wiley 2008.
Google Scholar -
Wu
S.,
Powers
S.,
Zhu
W.,
Hannun
Y.A.,
Substantial contribution of extrinsic risk factors to cancer development. Nature.
2016;
529
(7584)
:
43-7
.
View Article PubMed Google Scholar -
Fedewa
S.A.,
Sauer
A.G.,
Siegel
R.L.,
Jemal
A.,
Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev.
2015;
24
(4)
:
637-52
.
View Article PubMed Google Scholar -
Boughton
B.,
Gansler
T.,
Stefanek
M.,
Reduce Your Cancer Risk: Twelve Steps To A Healthier Life Springer Publishing Company 2010.
Google Scholar -
Medicine
I.,
Health
B.G.,
Gelband
H.,
Sloan
F.A.,
Countries
C.C.,
National Academies Press 2007.
Google Scholar -
Wardle
J.,
Robb
K.,
Vernon
S.,
Waller
J.,
Screening for prevention and early diagnosis of cancer. Am Psychol.
2015;
70
(2)
:
119-33
.
View Article PubMed Google Scholar -
Jin
Z.,
Jiang
W.,
Wang
L.,
Biomarkers for gastric cancer: progression in early diagnosis and prognosis (Review). Oncol Lett.
2015;
9
(4)
:
1502-8
.
View Article PubMed Google Scholar -
Barh
D.,
Carpi
A.,
Verma
M.,
Gunduz
M.,
Taylor & Francis 2014.
View Article Google Scholar -
Gearhart
S.,
Ahuja
N.,
Yang
S.C.,
Early Diagnosis and Treatment of Cancer Series: Colorectal Cancer: Expert ConsultElsevier Health Sciences 2010.
Google Scholar -
Mascarenhas
L.,
Felgenhauer
J.L.,
Bond
M.C.,
Villaluna
D.,
Femino
J.D.,
Laack
N.N.,
Pilot Study of Adding Vincristine, Topotecan, and Cyclophosphamide to Interval-Compressed Chemotherapy in Newly Diagnosed Patients With Localized Ewing Sarcoma: A Report From the Children's Oncology Group. Pediatr Blood Cancer.
2016;
63
(3)
:
493-8
.
View Article PubMed Google Scholar -
Hayat
M.A.,
Springer Netherlands 2010.
Google Scholar -
Brown
B.J.,
Adeleye
A.O.,
Ibeh
J.N.,
A prospective study on the causes of delayed diagnosis of childhood cancer in Ibadan, Nigeria. Pediatr Hematol Oncol.
2015;
32
(6)
:
365-73
.
View Article PubMed Google Scholar -
Tudor Car
L.,
Papachristou
N.,
Bull
A.,
Majeed
A.,
Gallagher
J.,
El-Khatib
M.,
Clinician-identified problems and solutions for delayed diagnosis in primary care: a PRIORITIZE study. BMC Fam Pract.
2016;
17
(1)
:
131
.
View Article PubMed Google Scholar -
Seneviratne
S.,
Campbell
I.,
Scott
N.,
Coles
C.,
Lawrenson
R.,
Treatment delay for Māori women with breast cancer in New Zealand. Ethn Health.
2015;
20
(2)
:
178-93
.
View Article PubMed Google Scholar -
Lo
P.C.,
Dahlberg
S.E.,
Nishino
M.,
Johnson
B.E.,
Sequist
L.V.,
Jackman
D.M.,
Delay of treatment change after pubive progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer.
2015;
121
(15)
:
2570-7
.
View Article PubMed Google Scholar -
DeSantis
C.E.,
Siegel
R.L.,
Sauer
A.G.,
Miller
K.D.,
Fedewa
S.A.,
Alcaraz
K.I.,
Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin.
2016;
66
(4)
:
290-308
.
View Article PubMed Google Scholar -
Siegel
R.L.,
Miller
K.D.,
Jemal
A.,
Cancer statistics, 2016. CA Cancer J Clin.
2016;
66
(1)
:
7-30
.
View Article PubMed Google Scholar -
Sattarahmady
N.,
Heli
H.,
Mehdizadeh
A.R.,
Yarmohammadi
H.,
Mortazavi
S.M.,
Evaluation of Serum Catalase Activity and Malondialdehyde Level as Stress Oxidative Biomarkers among Iranian Welders. Galen Med J.
2015;
4
(3)
:
62-6
.
-
Peters
K.E.,
Peters
K.E.,
Walters
C.C.,
Moldowan
J.,
Cambridge University Press 2005.
Google Scholar -
Sawyers
C.L.,
The cancer biomarker problem. Nature.
2008;
452
(7187)
:
548-52
.
View Article PubMed Google Scholar -
Verma
M.,
Springer 2012.
Google Scholar -
Ravelli
A.,
Reuben
J.M.,
Lanza
F.,
Anfossi
S.,
Cappelletti
M.R.,
Zanotti
L.,
Solid Tumor Working Party of European Blood
Marrow Transplantation Society (EBMT)
Breast cancer circulating biomarkers: advantages, drawbacks, and new insights. Tumour Biol.
2015;
36
(9)
:
6653-65
.
View Article PubMed Google Scholar -
Hermann
T.,
Patel
D.J.,
Adaptive recognition by nucleic acid aptamers. Science.
2000;
287
(5454)
:
820-5
.
View Article PubMed Google Scholar -
Brody
E.N.,
Gold
L.,
Aptamers as therapeutic and diagnostic agents. J Biotechnol.
2000;
74
(1)
:
5-13
.
View Article PubMed Google Scholar -
Tombelli
S.,
Minunni
M.,
Mascini
M.,
Analytical applications of aptamers. Biosens Bioelectron.
2005;
20
(12)
:
2424-34
.
View Article PubMed Google Scholar -
Nilsen-Hamilton
M.,
Aptamers in Bioanalysis. J Am Chem Soc.
2009;
131
(33)
:
12018
.
View Article Google Scholar -
Iliuk
A.B.,
Hu
L.,
Tao
W.A.,
Aptamer in bioanalytical applications. Anal Chem.
2011;
83
(12)
:
4440-52
.
View Article PubMed Google Scholar -
Mascini
M.,
Wiley 2009.
View Article Google Scholar -
Lyons
G.,
UCC 2006.
Google Scholar -
O'Sullivan
C.K.,
Aptasensors\textemdashthe future of biosensing?. Anal Bioanal Chem.
2002;
372
(1)
:
44-8
.
View Article PubMed Google Scholar -
Luzi
E.,
Minunni
M.,
Tombelli
S.,
Mascini
M.,
New trends in affinity sensing: aptamers for ligand binding. Trends Analyt Chem.
2003;
22
(11)
:
810-8
.
View Article Google Scholar -
Hianik
T.,
Wang
J.,
Electrochemical aptasensors\textendashrecent achievements and perspectives. Electroanalysis.
2009;
21
(11)
:
1223-35
.
View Article Google Scholar -
Chen
X.,
Zhang
Q.,
Qian
C.,
Hao
N.,
Xu
L.,
Yao
C.,
Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly(o-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag. Biosens Bioelectron.
2015;
64
:
485-92
.
View Article PubMed Google Scholar -
Robati
R.Y.,
Arab
A.,
Ramezani
M.,
Langroodi
F.A.,
Abnous
K.,
Taghdisi
S.M.,
Aptasensors for quantitative detection of kanamycin. Biosens Bioelectron.
2016;
82
:
162-72
.
View Article PubMed Google Scholar -
Cheng
L.,
Zhang
J.,
Lin
Y.,
Wang
Q.,
Zhang
X.,
Ding
Y.,
An electrochemical molecular recognition-based aptasensor for multiple protein detection. Anal Biochem.
2015;
491
:
31-6
.
View Article PubMed Google Scholar -
Sattarahmady
N.,
Rahi
A.,
Heli
H.,
A signal-on built in-marker electrochemical aptasensor for human prostate-specific antigen based on a hairbrush-like gold nanostructure. Sci Rep.
2017;
7
(1)
:
11238
.
View Article PubMed Google Scholar -
Negahdary
M.,
Behjati-Ardakani
M.,
Sattarahmady
N.,
Yadegari
H.,
Heli
H.,
Electrochemical aptasensing of human cardiac troponin I based on an array of gold nanodumbbells-Applied to early detection of myocardial infarction. Sens Actuators B Chem.
2017;
252
:
62-71
.
View Article Google Scholar -
Negahdary
M.,
Behjati-Ardakani
M.,
Sattarahmady
N.,
Heli
H.,
An Aptamer-based Biosensor for Troponin I Detection in Diagnosis of Myocardial Infarction. J Biomed Phys Eng.
2018;
8
(2)
:
167-78
.
View Article PubMed Google Scholar -
Negahdary
M.,
Behjati-Ardakani
M.,
Heli
H.,
An electrochemical troponin T aptasensor based on the use of a macroporous gold nanostructure. Mikrochim Acta.
2019;
186
(6)
:
377
.
View Article PubMed Google Scholar -
Negahdary
M.,
Heli
H.,
An ultrasensitive electrochemical aptasensor for early diagnosis of Alzheimer's disease, using a fern leaves-like gold nanostructure. Talanta.
2019;
198
:
510-7
.
View Article PubMed Google Scholar -
Qureshi
A.,
Roci
I.,
Gurbuz
Y.,
Niazi
J.H.,
Wiley Online Library 2015.
Google Scholar -
Zhao
J.,
He
X.,
Bo
B.,
Liu
X.,
Yin
Y.,
Li
G.,
A electrochemical aptasensor for simultaneous detection of two tumor markers. Biosens Bioelectron.
2012;
34
(1)
:
249-52
.
View Article PubMed Google Scholar -
Cho
H.,
Yeh
E.C.,
Sinha
R.,
Laurence
T.A.,
Bearinger
J.P.,
Lee
L.P.,
Single-step nanoplasmonic VEGF165 aptasensor for early cancer diagnosis. ACS Nano.
2012;
6
(9)
:
7607-14
.
View Article PubMed Google Scholar -
Amouzadeh Tabrizi
M.,
Shamsipur
M.,
Farzin
L.,
A high sensitive electrochemical aptasensor for the determination of VEGF(165) in serum of lung cancer patient. Biosens Bioelectron.
2015;
74
:
764-9
.
View Article PubMed Google Scholar -
Cennamo
N.,
Pesavento
M.,
Lunelli
L.,
Vanzetti
L.,
Pederzolli
C.,
Zeni
L.,
An easy way to realize SPR aptasensor: A multimode plastic optical fiber platform for cancer biomarkers detection. Talanta.
2015;
140
:
88-95
.
View Article PubMed Google Scholar -
Jolly
P.,
Formisano
N.,
Tká\vc
J.,
Kasák
P.,
Frost
C.G.,
Estrela
P.,
Label-free impedimetric aptasensor with antifouling surface chemistry: a prostate specific antigen case study. Sens Actuators B Chem.
2015;
209
:
306-12
.
View Article Google Scholar -
Liu
B.,
Lu
L.,
Hua
E.,
Jiang
S.,
Xie
G.,
Detection of the human prostate-specific antigen using an aptasensor with gold nanoparticles encapsulated by graphitized mesoporous carbon. Mikrochim Acta.
2012;
178
(1-2)
:
163-70
.
View Article Google Scholar -
Rahi
A.,
Sattarahmady
N.,
Heli
H.,
Label-free electrochemical aptasensing of the human prostate-specific antigen using gold nanospears. Talanta.
2016;
156-157
:
218-24
.
View Article PubMed Google Scholar -
Tahmasebi
F.,
Noorbakhsh
A.,
Sensitive Electrochemical Prostate Specific Antigen Aptasensor: Effect of Carboxylic Acid Functionalized Carbon Nanotube and Glutaraldehyde Linker. Electroanalysis.
2016;
28
(5)
:
1134-45
.
View Article Google Scholar -
Cha
T.,
Cho
S.,
Kim
Y.T.,
Lee
J.H.,
Rapid aptasensor capable of simply diagnosing prostate cancer. Biosens Bioelectron.
2014;
62
:
31-7
.
View Article PubMed Google Scholar -
Cai
S.,
Chen
M.,
Liu
M.,
He
W.,
Liu
Z.,
Wu
D.,
A signal amplification electrochemical aptasensor for the detection of breast cancer cell via free-running DNA walker. Biosens Bioelectron.
2016;
85
:
184-9
.
View Article PubMed Google Scholar -
Chang
K.,
Pi
Y.,
Lu
W.,
Wang
F.,
Pan
F.,
Li
F.,
Label-free and high-sensitive detection of human breast cancer cells by aptamer-based leaky surface acoustic wave biosensor array. Biosensors and Bioelectronics.
2014;
(60)
:
318-24
.
View Article Google Scholar -
Ilgu
M.,
Nilsen-Hamilton
M.,
Aptamers in analytics. Analyst (Lond).
2016;
141
(5)
:
1551-68
.
View Article PubMed Google Scholar -
Cai
S.,
Li
G.,
Zhang
X.,
Xia
Y.,
Chen
M.,
Wu
D.,
A signal-on fluorescent aptasensor based on single-stranded DNA-sensitized luminescence of terbium (III) for label-free detection of breast cancer cells. Talanta.
2015;
138
:
225-30
.
View Article PubMed Google Scholar -
Liang
L.,
Su
M.,
Li
L.,
Lan
F.,
Yang
G.,
Ge
S.,
Aptamer-based fluorescent and visual biosensor for multiplexed monitoring of cancer cells in microfluidic paper-based analytical devices. Sens Actuators B Chem.
2016;
229
:
347-54
.
View Article Google Scholar -
Li
Y.,
Zhang
Y.,
Zhao
M.,
Zhou
Q.,
Wang
L.,
Wang
H.,
A simple aptamer-functionalized gold nanorods based biosensor for the sensitive detection of MCF-7 breast cancer cells. Chem Commun (Camb).
2016;
52
(20)
:
3959-61
.
View Article PubMed Google Scholar -
Borghei
Y.S.,
Hosseini
M.,
Dadmehr
M.,
Hosseinkhani
S.,
Ganjali
M.R.,
Sheikhnejad
R.,
Visual detection of cancer cells by colorimetric aptasensor based on aggregation of gold nanoparticles induced by DNA hybridization. Anal Chim Acta.
2016;
904
:
92-7
.
View Article PubMed Google Scholar -
Yan
M.,
Sun
G.,
Liu
F.,
Lu
J.,
Yu
J.,
Song
X.,
An aptasensor for sensitive detection of human breast cancer cells by using porous GO/Au composites and porous PtFe alloy as effective sensing platform and signal amplification labels. Anal Chim Acta.
2013;
798
:
33-9
.
View Article PubMed Google Scholar -
Li
C.,
Meng
Y.,
Wang
S.,
Qian
M.,
Wang
J.,
Lu
W.,
Mesoporous Carbon Nanospheres Featured Fluorescent Aptasensor for Multiple Diagnosis of Cancer in Vitro and in Vivo. ACS Nano.
2015;
9
(12)
:
12096-103
.
View Article PubMed Google Scholar -
Wang
K.,
Zhang
R.,
Sun
N.,
Li
X.,
Wang
J.,
Cao
Y.,
Near-Infrared Light-Driven Photoelectrochemical Aptasensor Based on the Upconversion Nanoparticles and TiO2/CdTe Heterostructure for Detection of Cancer Cells. ACS Appl Mater Interfaces.
2016;
8
(39)
:
25834-9
.
View Article PubMed Google Scholar -
Yu
F.,
Li
G.,
Mao
C.,
An electrochemiluminescence aptasensor for tumor cells assay based on signal amplification of Ru (II) covalently doped silica nanoparticles. Electrochem Commun.
2011;
13
(11)
:
1244-7
.
View Article Google Scholar -
Liu
G.,
Mao
X.,
Phillips
J.A.,
Xu
H.,
Tan
W.,
Zeng
L.,
Aptamer-nanoparticle strip biosensor for sensitive detection of cancer cells. Anal Chem.
2009;
81
(24)
:
10013-8
.
View Article PubMed Google Scholar -
van Horssen
R.,
Ten Hagen
T.L.,
Eggermont
A.M.,
TNF-α in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist.
2006;
11
(4)
:
397-408
.
View Article PubMed Google Scholar -
Liu
Y.,
Zhou
Q.,
Revzin
A.,
An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst (Lond).
2013;
138
(15)
:
4321-6
.
View Article PubMed Google Scholar -
Liu
L.,
Liu
F.,
Jiang
D.,
Xiang
G.,
Liu
C.,
Yang
J.,
Hybridization chain reaction and target recycling enhanced tumor necrosis factor alpha aptasensor with host-guest interaction for signal probe collection. Sens Actuators B Chem.
2016;
231
:
680-7
.
View Article Google Scholar -
Cao
L.,
Cheng
L.,
Zhang
Z.,
Wang
Y.,
Zhang
X.,
Chen
H.,
Visual and high-throughput detection of cancer cells using a graphene oxide-based FRET aptasensing microfluidic chip. Lab Chip.
2012;
12
(22)
:
4864-9
.
View Article PubMed Google Scholar -
Shi
H.W.,
Wu
M.S.,
Du
Y.,
Xu
J.J.,
Chen
H.Y.,
Electrochemiluminescence aptasensor based on bipolar electrode for detection of adenosine in cancer cells. Biosens Bioelectron.
2014;
55
:
459-63
.
View Article PubMed Google Scholar -
Feng
L.,
Chen
Y.,
Ren
J.,
Qu
X.,
A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials.
2011;
32
(11)
:
2930-7
.
View Article PubMed Google Scholar -
Wang
T.,
Liu
J.,
Gu
X.,
Li
D.,
Wang
J.,
Wang
E.,
Label-free electrochemical aptasensor constructed by layer-by-layer technology for sensitive and selective detection of cancer cells. Anal Chim Acta.
2015;
882
:
32-7
.
View Article PubMed Google Scholar -
Zhang
X.,
Xiao
K.,
Cheng
L.,
Chen
H.,
Liu
B.,
Zhang
S.,
Visual and highly sensitive detection of cancer cells by a colorimetric aptasensor based on cell-triggered cyclic enzymatic signal amplification. Anal Chem.
2014;
86
(11)
:
5567-72
.
View Article PubMed Google Scholar -
Tian
C.Y.,
Xu
J.J.,
Chen
H.Y.,
A novel aptasensor for the detection of adenosine in cancer cells by electrochemiluminescence of nitrogen doped TiO2 nanotubes. Chem Commun (Camb).
2012;
48
(66)
:
8234-6
.
View Article PubMed Google Scholar -
He
Y.,
Lin
Y.,
Tang
H.,
Pang
D.,
A graphene oxide-based fluorescent aptasensor for the turn-on detection of epithelial tumor marker mucin 1. Nanoscale.
2012;
4
(6)
:
2054-9
.
View Article PubMed Google Scholar -
Liu
X.,
Qin
Y.,
Deng
C.,
Xiang
J.,
Li
Y.,
A simple and sensitive impedimetric aptasensor for the detection of tumor markers based on gold nanoparticles signal amplification. Talanta.
2015;
132
:
150-4
.
View Article PubMed Google Scholar -
Cao
H.,
Ye
D.,
Zhao
Q.,
Luo
J.,
Zhang
S.,
Kong
J.,
A novel aptasensor based on MUC-1 conjugated CNSs for ultrasensitive detection of tumor cells. Analyst (Lond).
2014;
139
(19)
:
4917-23
.
View Article PubMed Google Scholar -
Shi
G.F.,
Cao
J.T.,
Zhang
J.J.,
Huang
K.J.,
Liu
Y.M.,
Chen
Y.H.,
Aptasensor based on tripetalous cadmium sulfide-graphene electrochemiluminescence for the detection of carcinoembryonic antigen. Analyst (Lond).
2014;
139
(22)
:
5827-34
.
View Article PubMed Google Scholar -
Wang
Y.L.,
Cao
J.T.,
Chen
Y.H.,
Liu
Y.M.,
A label-free electrochemiluminescence aptasensor for carcinoembryonic antigen detection based on electrodeposited ZnSCdS on MoS 2 decorated electrode. Anal Methods.
2016;
8
(26)
:
5242-7
.
View Article Google Scholar -
Wu
Z.,
Li
H.,
Liu
Z.,
An aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Sens Actuators B Chem.
2015;
206
:
531-7
.
View Article Google Scholar -
Liu
Z.,
Wang
Y.,
Guo
Y.,
Dong
C.,
Label-free Electrochemical Aptasensor for Carcino-embryonic Antigen Based on Ternary Nanocomposite of Gold Nanoparticles, Hemin and Graphene. Electroanalysis.
2015
.
View Article Google Scholar -
Verberne
C.J.,
Wiggers
T.,
Vermeulen
K.M.,
de Jong
K.P.,
Detection of recurrences during follow-up after liver surgery for colorectal metastases: both carcinoembryonic antigen (CEA) and imaging are important. Ann Surg Oncol.
2013;
20
(2)
:
457-63
.
View Article PubMed Google Scholar -
Verberne
C.J.,
Zhan
Z.,
Heuvel
E. van den,
Grossmann
I.,
Doornbos
P.M.,
Havenga
K.,
Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized trial. Eur J Surg Oncol.
2015;
41
(9)
:
1188-96
.
View Article PubMed Google Scholar -
Z. Liu,
Wang
Y.,
Guo
Y.,
Dong
C.,
Label‐free Electrochemical Aptasensor for Carcino‐embryonic Antigen Based on Ternary Nanocomposite of Gold Nanoparticles, Hemin and Graphene. Electroanalysis.
2015;
28
(5)
:
1023-1028
.
View Article Google Scholar -
Falcone
F.,
Sabbatani
S.,
Fini
A.,
Turba
E.,
Magri
P.,
D'Ales
A.,
Combination of tissue polypeptide antigen (TPA) and carcino embryonic antigen (CEA) in different types of cancer. Nucl Med Commun.
1985;
6
(5)
:
299-304
.
View Article PubMed Google Scholar -
Loo
F.C.,
Ng
S.P.,
Wu
C.M.,
Kong
S.K.,
An aptasensor using DNA aptamer and white light common-path SPR spectral interferometry to detect cytochrome-c for anti-cancer drug screening. Sens Actuators B Chem.
2014;
198
:
416-23
.
View Article Google Scholar -
Lavrik
I.N.,
Springer: New York; 2012.
Google Scholar -
Yuan
B.,
Zhou
Y.,
Guo
Q.,
Wang
K.,
Yang
X.,
Meng
X.,
A signal-on split aptasensor for highly sensitive and specific detection of tumor cells based on FRET. Chem Commun (Camb).
2016;
52
(8)
:
1590-3
.
View Article PubMed Google Scholar -
Zamay
G.S.,
Zamay
T.N.,
Kolovskii
V.A.,
Shabanov
A.V.,
Glazyrin
Y.E.,
Veprintsev
D.V.,
Electrochemical aptasensor for lung cancer-related protein detection in crude blood plasma samples. Sci Rep.
2016;
6
(1)
:
34350
.
View Article PubMed Google Scholar -
Sato
K.,
Nakate
H.,
Sogabe
S.,
Kohno
H.,
Maeda
K.,
Konno
M.,
Watanabe
S.,
Clinical Chemistry Amer Assoc Clinical Chemistry: Washington, DC 20037-1526; 1987.
Google Scholar -
Hashemian
Z.,
Khayamian
T.,
Saraji
M.,
Shirani
M.P.,
Aptasensor based on fluorescence resonance energy transfer for the analysis of adenosine in urine samples of lung cancer patients. Biosens Bioelectron.
2016;
79
:
334-40
.
View Article PubMed Google Scholar -
Qureshi
A.,
Gurbuz
Y.,
Niazi
J.H.,
Label-free capacitance based aptasensor platform for the detection of HER2/ErbB2 cancer biomarker in serum. Sens Actuators B Chem.
2015;
220
:
1145-51
.
View Article Google Scholar -
Hu
Y.,
Li
L.,
Guo
L.,
The sandwich-type aptasensor based on gold nanoparticles/DNA/magnetic beads for detection of cancer biomarker protein AGR2. Sens Actuators B Chem.
2015;
209
:
846-52
.
View Article Google Scholar -
Chen
X.,
Pan
Y.,
Liu
H.,
Bai
X.,
Wang
N.,
Zhang
B.,
Label-free detection of liver cancer cells by aptamer-based microcantilever biosensor. Biosens Bioelectron.
2016;
79
:
353-8
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 7 (2019)
Page No.: 3315-3324
Published on: 2019-07-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7234 times
- Download PDF downloaded - 1512 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress