Abstract
Abstract: Acute Myeloid Leukemia (AML) is a malignant hematopoietic disease caused by the presence of a malignant clone in the bone marrow. The classic AML treatment includes a combination of an Anthracycline and Cytarabine. This study aimed to evaluate the effect of high doses of Daunorubicin on patients' outcome.
Methods: During the study period, 16 AML patients received induction therapy with Cytarabine (100 mg/m2/d) for 7 days and Daunorubicin (90 mg/m2/d) for 3 days. Outcome analysis was performed to evaluate the overall survival (OS) and disease-free survival (DFS) during 2 years of study.
Results: The mean age of patients was 38+/-12.38 years, with the age range between 16 and 54 years old. Seven patients (43.8%) were females, and 9 cases (56.3%) were males. OS was 81.3%, with a mean of 396.88 days. (95% CI: 306.99-486.77). DFS was 83.3%, with a mean of 383.57 days (95% CI: 299.88-467.26). The log-rank test showed a significant difference in DFS of AML sub-types, as M1 subtypes had lower DFS (P log-rank= 0.013). Although M1 subtypes had a lower OS, there was no significant difference in OS between subgroups (P log-rank= 0.067).
Conclusion: Although disease-free survival was improved by increasing the dose of daunorubicin, there was no difference in the overall survival between the AML subgroups and sexes.
Introduction
Acute Myeloid Leukemia (AML) is a malignant hematopoietic tumor of the bone marrow myeloid cells1,2,3, which results in the aggregation of malignant cells in the bone marrow and the onset of anemia, neutropenia, thrombocytopenia1,2,3,4,5,6,7,8. The incidence of AML is more common in men, and the ratio of incidence in men to women is 3 to 2. The incidence of AML increases with aging9,10,11,12.
Several etiologies are associated with AML incidence, including dysplastic diseases, congenital diseases (Down syndrome, Fanconi anemia), and environmental exposures (ionizing radiation, chemical exposures, and history of chemotherapy)13,14,15. In the absence of treatment, the median survival of patients is about 11 to 20 weeks16.
AML treatment includes induction therapy (Remission Induction) and Post Remission Induction. The aim of Remission Induction is to achieve a complete response to treatment (Complete Remission). Despite recent improvements, the AML cure rate increased from 20% in the 1960s — 1980s, to 40-70% in 2000. AML standard treatment includes an Anthracycline and Cytarabine combination 17.
The most widely used AML induction chemotherapy regimen is Cytarabine as a continuous infusion of 100-200 mg/m2 for 7 days and intravenous Anthracycline for 3 days. Different dosages have been performed using Daunorubicin (30 mg/m2/d to 90 mg/m2/day) in other studies.
This study aimed to determine the overall survival and disease- free survival in AML patient treated with 90 mg/m2/day of Daunorubicin.
Materials - Methods
This cross-sectional study was conducted between December 2015 to September 2017 in the Department of Hematology and Oncology, Tabriz University of Medical Sciences. This study has been approved as a confirmed research proposal (94/13) and obtaining the permission of the Ethics Committee of Tabriz University of Medical Sciences (TBZMED.REC.1394.793). Ethical consent forms were obtained from a ll patients.
Inclusion criteria
All patients aged 15-60 years with newly diagnosed AML and other than AML (M3), based on morphological and flow cytometric criteria, and patients with a cardiac ejection fraction of more than 50% were included in this study.
Exclusion criteria
Patients during pregnancy and breastfeeding, or having malignancies and secondary or previously treated for AML, and patients with a cardiac ejection fraction of less than 50% were excluded.
After confirmation of the diagnosis of AML according to WHO diagnostic criteria (presence of more than 20% of myeloblasts in bone marrow)7, patients were treated with an induction regimen of 7 + 3. Morphological evaluation of cells, immune-histo-chemical staining, flow cytometric characteristics, and cytogenetic analysis for patients were performed.
Patients were treated with Cytarabine (Ebewe, Austria) 100 mg/m2 as a 24-hour intravenous infusion for 7 days, and Daunorubicin 90 mg/m2 (Pfizer, Germany) was infused intravenously for three days18. At the 30th day after treatment started, patients were evaluated for response to treatment by bone marrow aspiration.
In the case of complete remission (19), which included neutrophil count over 1000, platelet count above 100,000, absence of premature cells in peripheral blood and less than 5% in bone marrow, and absence of Auerrod, patients were treated with Cytarabine (100 mg/m2) as infusion during 24-hour for 5 days, and 45 mg/m2 of Daunorubicin as intravenous infusion for two days. This treatment was repeated with an interval of one month, and patients underwent monthly blood tests until relapse or death due to non-AML.
Statistical analysis
Prognostic outcomes were assessed as overall survival (OS) and disease - free survival (DFS). Overall survival was determined at the time of diagnosis and the date of death or the last follow-up. DFS was evaluated at the time interval between the diagnosis date and the date of the first relapse or the last follow-up.
The survival analysis was performed using the Kaplan-Meier method and the log-rank test for evaluating the differences between mentioned factors. Data analysis was performed by SPSS software version 21.
Results
Sixteen patients were recruited in this study, including 7 (43.8%) females and 9 (56.2%) males. The patients were between 16-54 years old, and their mean age was 38 ± 12.38 years. The most common detected sub-type was AML, M2 (n=9, 56.25%). Table 1 shows the frequency of AML different subtypes (Table 1).
| AML Subtype | Frequency | Percent |
|---|---|---|
| M0 | 1 | 6.25% |
| M1 | 3 | 18.75% |
| M2 | 9 | 56.25% |
| M4 | 3 | 18.75% |
| M5 | - | - |
The median white blood cell count was 2125.0/cm3 (800 to 83480), with mean hemoglobin levels of 5.88 ± 2.66 g/dl, and the median platelet count was 29500.0/mm3 (5000 to 42000).
In 13 patients (81.3%), fever was reported, and mucositis was detected in 10 patients (62.5 %). The highest and lowest neutropenic periods in patients were 8 days and 26 days, respectively. The median duration of neutropenia was 23 days. The maximum and minimum days for antibiotic treatment were 8 days and 26 days, respectively, and the median duration of antibiotic therapy was 8 days. From 16 AML patients, only one patient had evidence of cardiac function reduction. Eleven AMLs (68.8%) had complete remission, and there was no significant difference between the type of AML and complete remission response rate (P = 0.5).
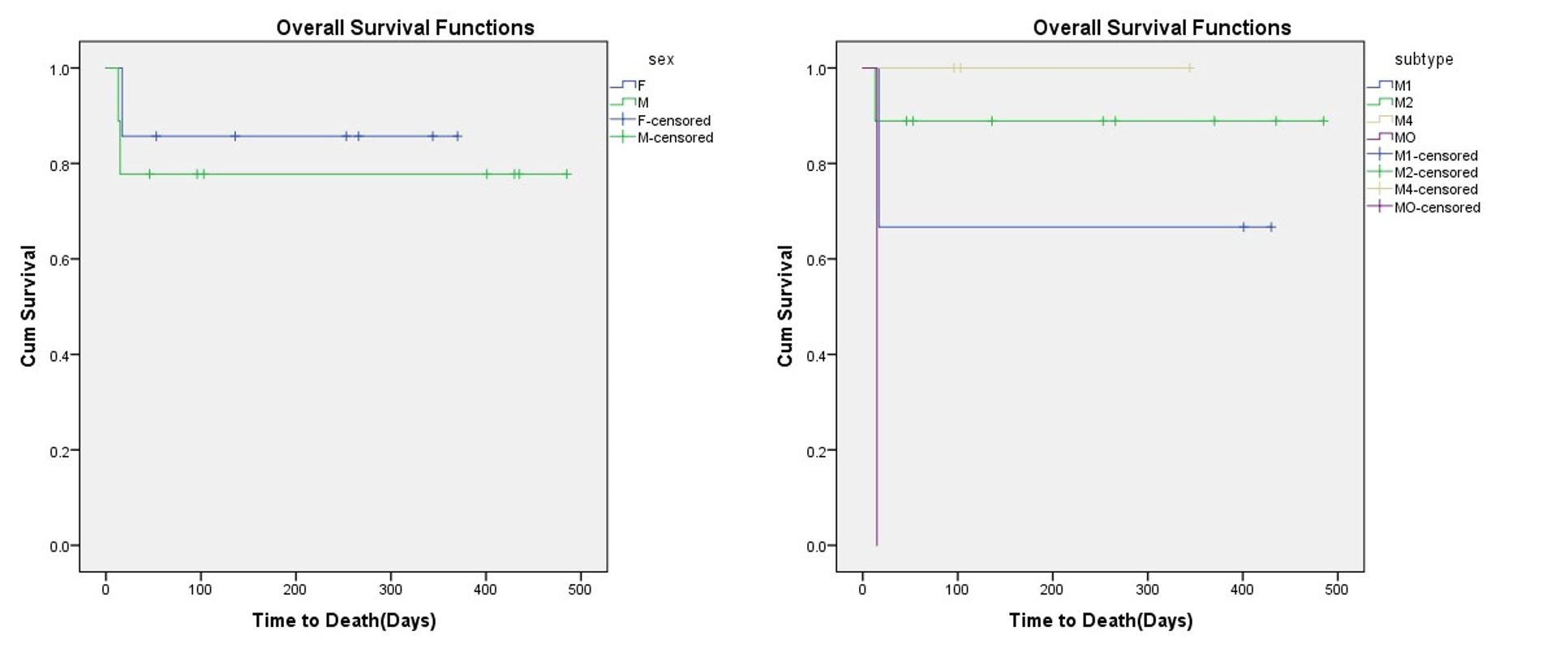
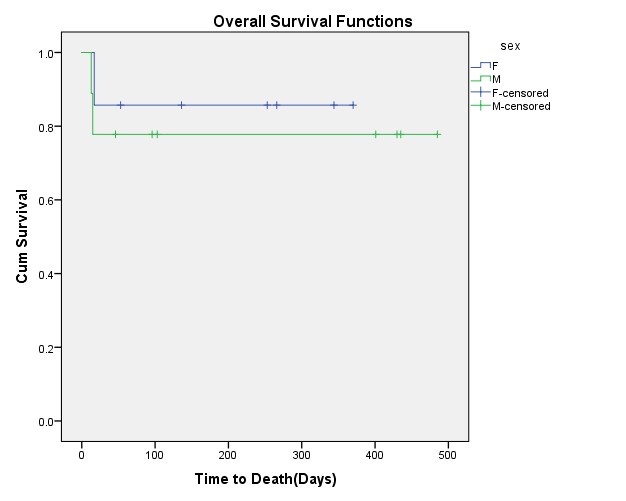
The mean follow-up time was 216.44 ± 178.08 days in 16 AML patients. The overall survival was 81.3%, with the mean OS of 396.88 days (95% CI: 306.261-486.765). The highest overall survival was observed for M2 sub-type, but there was no significant difference in prognosis between different AML sub-types (P Log-rank = 0.0 67). Although male patients had lower OS than females (77.8% vs. 85.7%), this difference was not significant (P Log-rank=0.640) (Figure 1).
The disease-free survival (DFS) was 83.3%, with a mean of 383.57 days (95% CI: 299.88 – 467.26). None of the M2 sub-types had relapsed during the study period, and so there was a significant difference in DFS between AML sub-types (P Log-rank=0.013). From each of the male and female AML cases, one patient had relapsed (P Log-rank= 0.728) (Figure 2).
Discussion
We followed 16 AML patients in two years to evaluate the prognostic effect of treatment with 90 mg/m2/day of Daunorubicin. The most common detected sub-type was AML, M2 (n=9, 56.25%), and there was a significant difference of DFS between different sub-types, while none of the M2 sub-types had relapsed.
Recently Lee et al. (2016) showed the effectiveness of increasing Daunorubicin doses to 90 mg/m2/day compared to 45 mg/m2/day dosage, in the form of 7+3 protocol in patients younger than 60 years.
The overall remission rate was 70.6% vs. 57.33% (P<0.001), and the overall survival rate was 23.7 months, compared to 15.7 months (P=0.005)18. In another study, the complete remission rate was 82.5% vs. 72%, in the groups receiving the same protocols. Overall survival rate (46.8% vs. 34.6%) and the disease survival rate (8.8% and 28.4%) were significantly higher in AMLs, who received 90 mg/m2/day Daunorubicin19.
In Löwenberg et al. study, complete remission (CR) was 73% in AMLs receiving 90 mg/m2 of Daunorubicin, compared with 51% CR of the standard care with 45 mg/m2 (P = 0.08). The overall survival (38% vs. 23% P=0.16) was not significantly different between the two groups20.
Appelbaum et al. evaluated AML patients treated with a standard dose (45 mg/m2) and suggested dose (90 mg/m2) of Daunorubicin19. Their results showed that high dose Daunorubicin significantly increased patient's survival (P=0.0003). We could not find any significant difference in OS between different AML sub-types, but there was a significant DFS between groups. Considering the controversial results of different studies, further studies with better study designs and larger sample size are required to demonstrate the effective response to high doses (90 mg/m2) of Daunorubicin.
Conclusion
In this study, there was not any significant relationship between AML sub-types and overall survival in patients with acute myeloid leukemia, which was due to the small size in the study. In the case of a multicentre study, improvement in the results may be achieved by increasing the sample size.
Abbreviations
AML: Acute Myeloid Leukemia
CI: Confidence Interval
CR: Complete Remission
DFS: Diseases Free Survival
OS: Overall Survival
WHO: World Health Organization
Author Contributions
1) R.D., E.I.J., and Z.S. made substantial contributions to the conception and design of the work; and the acquisition, analysis, interpretation of data;
2) A.N., A.E., S.H.C., B.N. made data extraction and management;
3) R.D., E.I.J., and Z.S. drafted the work or revised it critically for important intellectual content;
4) All authors approved the version to be published; and
5) All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
None of the authors of this article have conflicting interests.
References
-
T J Ley,
C Miller,
L Ding,
B J Raphael,
A J Mungall,
A Robertson,
Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine.
2013 May 30;
368
(22)
:
2059-74
.
View Article PubMed Google Scholar -
Dohner
H,
Weisdorf
D J,
Bloomfield
C D,
Acute Myeloid Leukemia. The New England journal of medicine.
2015 ;
373
(12)
:
1136-52
.
View Article PubMed Google Scholar -
Estey
E H,
Acute myeloid leukemia: 2013 update on risk-stratification and management. American journal of hematology.
2013;
88
(4)
:
318-27
.
View Article PubMed Google Scholar -
Estey
E,
Dohner
H,
Acute myeloid leukaemia. Lancet.
2006 Nov 25;
368
(9550)
:
1894-907
.
View Article PubMed Google Scholar -
O'Donnell
M R,
Abboud
C N,
Altman
J,
Appelbaum
F R,
Arber
D A,
Attar
E,
NCCN Clinical Practice Guidelines Acute myeloid leukemia. Journal of the National Comprehensive Cancer Network : JNCCN.
2012 Aug;
10
(8)
:
984-1021
.
View Article PubMed Google Scholar -
Rubnitz
J E,
Gibson
B,
Smith
F O,
Acute myeloid leukemia. Hematology/oncology clinics of North America.
2010 Feb;
24
(1)
:
35-63
.
View Article PubMed Google Scholar -
R M Stone,
O'Donnell
M R,
Sekeres
M A,
Acute myeloid leukemia. Hematology American Society of Hematology Education Program.
2004;
:
98-117
.
PubMed Google Scholar -
Su
L,
Zhu
X,
Gao
S,
Li
W,
Liu
X,
Tan
Y,
High-dose versus standard-dose daunorubicin in induction therapy for young patients with de novo acute myeloid leukaemia: a meta-analysis of randomised trials. Journal of chemotherapy.
2016 Apr;
28
(2)
:
123-8
.
View Article PubMed Google Scholar -
Creutzig
U,
Dworzak
M N,
Bochennek
K,
Faber
J,
Flotho
C,
Graf
N,
First experience of the AML-Berlin-Frankfurt-Munster group in pediatric patients with standard-risk acute promyelocytic leukemia treated with arsenic trioxide and all-trans retinoid acid. . Pediatric blood & cancer.
2017;
64
(8)
.
View Article PubMed Google Scholar -
Luskin
M R,
Lee
J W,
Fernandez
H F,
O Abdel-Wahab,
Bennett
J M,
Ketterling
R P,
Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood.
2016;
127
(12)
:
1551-8
.
View Article PubMed Google Scholar -
Vaezi
M,
Bahar
B,
Mousavi
A,
Yaghmai
M,
Kasaeian
A,
Souri
M,
Comparison of 60 and 80 mg/m(2) of daunorubicin in induction therapy of acute myeloid leukaemia. Hematological oncology.
2017;
35
(1)
:
101-5
.
View Article PubMed Google Scholar -
Wingo
P A,
Cardinez
C J,
Landis
S H,
Greenlee
R T,
Ries
L A,
Anderson
R N,
Long-term trends in cancer mortality in the United States, 1930-1998. Cancer.
2003;
97
(12)
:
3133-275
.
View Article PubMed Google Scholar -
V M Aquino,
Acute myelogenous leukemia. Current problems in pediatric and adolescent health care.
2002;
32
(2)
:
50-8
.
View Article PubMed Google Scholar -
Deschler
B,
Lubbert
M,
Acute myeloid leukemia: epidemiology and etiology. Cancer.
2006;
107
(9)
:
2099-107
.
View Article PubMed Google Scholar -
Pogoda
J M,
Preston-Martin
S,
Smoking and risk of acute myeloid leukemia in adults. Cancer causes & control.
2006 Apr;
17
(3)
:
351-2
.
View Article PubMed Google Scholar -
Sekeres
M A,
Stone
R M,
Zahrieh
D,
Neuberg
D,
Morrison
V,
Angelo
D J De,
Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia.
2004 Apr;
18
(4)
:
809-16
.
View Article PubMed Google Scholar -
Tallman
M S,
Gilliland
D G,
Rowe
J M,
Drug therapy for acute myeloid leukemia. Blood.
2005;
106
(4)
:
1154-63
.
View Article PubMed Google Scholar -
Lee
J H,
Joo
Y D,
Kim
H,
Bae
S H,
Kim
M K,
Zang
D Y,
A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood.
2011 Oct 6;
118
(14)
:
3832-41
.
View Article PubMed Google Scholar -
Appelbaum
F R,
Gundacker
H,
Head
D R,
Slovak
M L,
Willman
C L,
Godwin
J E,
Age and acute myeloid leukemia. Blood.
2006 May 1;
107
(9)
:
3481-5
.
View Article PubMed Google Scholar -
Lowenberg
B,
Ossenkoppele
G J,
Putten
W Van,
Schouten
H C,
Graux
C,
Ferrant
A,
High-dose daunorubicin in older patients with acute myeloid leukemia. The New England journal of medicine.
2009;
361
(13)
:
1235-48
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 9 (2019)
Page No.: 3347-3351
Published on: 2019-09-10
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3777 times
- View Article downloaded - 0 times
- Download PDF downloaded - 1062 times
 Biomedpress
Biomedpress