Abstract
Introduction: Nicotine is the most important alkaloid compound in tobacco and is a major risk factor in the development of functional disorders of several organ systems. Some plants produce Curcuma longa (curcumin), which has antioxidant and neuro-protective properties.
Methods: This study was designed to evaluate the therapeutic effects of curcumin against nicotine injury on the hippocampus CA1 region of rats. In this study, 48 male rats were randomly assigned to eight groups: Normal control (saline) group, Nicotine control group (0.5 mg/kg); Curcumin groups (10, 30, and 60 mg/kg) and Nicotine + Curcumin groups (10, 30, and 60 mg/kg). Treatments were administered intra-peritoneally daily for 28 days. Golgi staining technique investigated the number of dendritic spines. Cresyl violet staining method was used to determine the number of neurons in the hippocampal region CA1. Griess technique was assessed to determine serum nitrite oxide levels. Also, the ferric reducing/antioxidant power (FRAP) method was applied to determine the total antioxidant capacity.
Results: Nicotine administration significantly increased the nitrite oxide level and decreased total antioxidant capacity. The number of neuronal dendritic spines, as well as neurons per se, also decreased, compared to the control group (p < 0.01). In all the members of the Curcumin and Nicotine + Curcumin groups, the number of neurons, neuronal dendritic spines and total antioxidant capacity increased significantly compared to the nicotine control group, while nitrite oxide level decreased significantly compared to the nicotine control group (p < 0.01).
Conclusions: It seems that Curcumin administration improved hippocampal CA1 region injury in rats caused by Nicotine.
INTRODUCTION
Tobacco consumption has declined dramatically in industrialized countries over the past three decades due to raising awareness of the risks to health and effective control policies, while it has increased in developing countries over the same period 1. Each cigarette has an average of 10-14 mg of nicotine2. Nicotine spreads rapidly into brain tissue through pulmonary circulation and within 10-20 seconds, attaches itself to the nicotinic acetylcholine receptors (nAChRs) 3. Nicotine in a cigarette is an alkaloid that passes through the blood-brain barrier and stimulates the mesolimbic dopamine system 4. Nicotine can regulate brain neurotransmitters, including catecholamine, serotonin, glutamate, and Gamma-Aminobutyric Acid (GABA). This substance also reduces the activity of superoxide dismutase, glutathione peroxidase, and glutathione reductase in the hippocampus 5. However, nicotine can induce oxidative stress in some organs, including the brain 3. Pathologic changes associated with neuronal apoptosis have been reported due to the use of nicotine 6. Cigarette smoking can cause hypoxia, cerebral ischemia, hemorrhage, atrophy, ataxia, cerebral infarction, and subarachnoid hemorrhage 7. Also, nicotine can induce increased oxidative stress and neuronal apoptosis, destroy Deoxyribonucleic acid (DNA), produce reactive oxygen species (ROS), and increase the production of lipid peroxidation8. Nicotine seems to activate the areas of the brain that play an essential role in drug addiction and learning process 9. Among the brain areas where nicotine has the greatest effect, is the mesocorticolimbic region (that contains nucleus accumbens), ventral tegmental area, the hippocampus, and the amygdala. In this region, the amygdala and hippocampus have structures that play an important role in the formation of long-term memory, and their function is associated with a stimulation reward system 10. Hippocampus is a part of the limbic system and seems to be essential for the formation of various types of learning and memory 11. Cornu Ammonis 1 (CA1) area, which belongs to the hippocampus, plays an essential role in converting short-term memory to long-term memory 12. In recent years, an increase is seen in the use of herbal medicines for reducing the side effects of drug use 13. For centuries, India and China have been using turmeric as an anti-inflammatory agent in the treatment of colic pain, toothache, chest pain, jaundice, anorexia, and menstrual problems 14. The rhizome extract contains mainly curcumin and, to a lesser extent, desmethoxycurcumin, and bisdemethoxycurcumin11. Curcumin has antioxidant and anti-inflammatory properties 15. The results of a study by Jayaprakasha et al. showed that in Alzheimer’s experimental model, curcumin could improve memory via intravenous injection of streptozotocin16. Curcumin can increase neurogenesis and improve cognitive processes in elderly female rats 17. The results of the study by Mashayekhi et al. on various antioxidants showed that curcumin is much more potent in breaking down free radicals than vitamin E. In addition, it can protect the brain against lipid peroxidation and break down nitrite oxide (NO)-induced radicals 18. Curcumin seems to prevent the effects of nicotine in the brain via the reduction of the oxidative stress mechanism. Considering effects of nicotine toxicity on the brain and the therapeutic properties of curcumin, the present study investigates the effects of curcumin on nicotine-induced toxicity in the hippocampus CA1 region of male rats and evaluates the antioxidant effect of curcumin on CA1 damage.
MATERIALS - METHODS
Animals
A total of 48 male Wistar rats) weighing 220-250 g (were purchased from the Pasteur Institute and transferred to the animal house in medical school. During the study, the animals were kept under standard conditions for 12-h light/12-h dark and 22 ± 2 ℃, on a straw bed in special cages. Water and food were available to the animals ad libitum. Standard food was including plate and treated municipal water was used to feed the animals. All investigations were approved by the Ethics Committee of Kermanshah University of Medical Sciences and conformed to the ethical and human principles of research (ethics certificate No.1396.562) 15.
Study groups and treatment of animals
Curcumin powder (Sigma, USA) with a chemical formula of 4-(OH)-3-(CH₃O) was dissolved in 0.9% normal saline solution to obtain relevant doses. The mixture of the solute and solvent was heated up to 70-80°C. The heterogeneous mixture was filtered to get a homogenous solution of curcumin in normal saline. A vial of nicotine (Sigma, USA) with a dose of 0.5 mg per kg of body weight was dissolved in 0.9% normal saline solution. Next, 48 male rats were randomly divided into 8 groups, and 6 rats were placed in each group. The first group (i.e., the normal control group) received normal saline through intra-peritoneal injection equivalent to the number of experimental groups. In the second group (i.e., the control group of nicotine), each animal received 0.5 mg/kg single dose course of nicotine, intraperitoneally. The solvent of nicotine was normal saline 16. Group 3, 4, and 5 were treated with curcumin. In these groups, each animal received 10, 30, and 60 mg/kg of curcumin intra-peritoneally for 28 days at 10 a.m. Group 6, 7 and 8 were Nicotine + Curcumin administration groups, wherein each animal received a single dose of 0.5 mg/kg Nicotine in order to induce damage. Then, they received 10, 30, and 60 mg/kg of curcumin intra-peritoneally for 28 days at 10 am as previously described 12,8.
Transcardiac perfusion
The transcardiac method was used for fixation. Around 24 h after the last injection of the drug, animals were intra-peritoneally anesthetized with ketamine 70 mg/kg and diazepam 10 mg/kg. The chest was opened in the midline. Following the completion of thoracotomy, the apex of the left ventricle was pierced, and a glass cannula of 1 mm diameter was inserted and fixed on ascending aorta. The pericardium and the right ventricle were cut. The left ventricle pathway was cut, and the ascending aorta was connected to a plastic tube via the glass cannula and descending aorta was clamped right above the diaphragm. The cannula linked to the normal saline solution was implanted into the aorta through an incision in the left ventricle. The descending aorta was fastened and, after washing the brain, the solution was removed through the incision made in the right atrium. Formalin 5% and buffer phosphate 7% were inoculated into the brain by the cannula, and the brain was fixed in 15 min. After perfusion, the brains were separated from the skull and stored in the same perfusion solution for three days as previously described 11.
Tissue preparing and Golgi methods
The Golgi method was used to observe neuron dendrites in hippocampus CA1 region. The Golgi method was applied using potassium dichromate, followed by silver nitrate. After brain fixation, tissue blocks were submerged in 3% potassium dichromate solution for 48 hours in a dark environment. The blocks were washed in 0.75% silver nitrate solution and then were submerged in the solution for 72 hours. Then, the tissues were washed in 1% silver nitrate solution. After that, preparing paraffin-embedded blocks were made, using the Automatic Tissue Processor. The steps of this process included fixation with 10% normal saline (for 72 hours), washing thoroughly under running water, dehydrating by raised doses of ethanol (50, 60, 70, 80, 90 and 100%, which included 3 min for each step and 100% ethanol step was repeated for three times), clearing by xylene (10 minutes for three times) and embedding in soft paraffin (three times, 15 min each time). At this stage, 5- µm coronal histological thin sections were cut from paraffin-embedded blocks, undertaken by a microtome instrument (Leica RM 2125, Leica Microsystems Nussloch GmbH; Germany), and 5 sections per animal were chosen. For the unification of the section selection, the first section was the 4th, and the last was the 24th (5 sections interval). Finally, the routine protocol for Golgi methods was implemented. At the end of the tissue processing, stained sections were mounted by entalan glue and assessed under Olympus BX-51T-32E01 research microscope, connected to a DP12 Camera with 3.34-million pixel resolution and Olysia Bio software (Olympus Optical Co. LTD, Tokyo, Japan) 12.
Cresyl violet method
The Cresyl violet staining method was used to determine the number of live cells in the hippocampus CA1 region. Six rat heads from each group, and five tissue samples from each mouse were selected for staining. After creating 5 µm cuts by microtome and performing tissue processing, the left hemispheres were stained, using Cresyl violet staining technique. Once the photo was prepared, the number of cells was counted per 1 mm2 area. On the slides, stained by means of Cresyl violet technique, the round cells without peak nose were considered as live cells 19.
Morphometrically technique
On the slides, marked via Golgi staining method, completely stained neurons with cell bodies in the central part of the tissue sections, distant from the surrounding stained neurons were included in the study. The dendritic tree of pyramidal neurons was revealed through camera lucida with magnification = 750. The dendritic exclusion order from the cell body was used for counting dendritic sections. Additionally, the Sholl procedure was applied to assess the concentration of dendritic divisions. In the slips, marked via Cresyl violet method, the round cells without peak nose were considered as live cells. The slides were imaged by Motic microscope, and cells were counted by Image J software 1.45 11.
Griess technique
Griess technique uses zinc sulfate powder to eliminate the serum protein of the samples. In this study, zinc sulfate powder (6 mg) was mixed with serum samples (400 μl) and vortexed for 1 min. The samples were centrifuged at 4 ℃ for 10 min at 12,000 rpm, and the supernatant was collected to measure nitrite oxide content. Briefly, 50 μl of sample was added to 100 μl of Griess reagent (Sigma; USA), and the reaction mixture was incubated for 30 min at room temperature. Sodium nitrite (0.1 M) was used for the standard curve, and increasing concentrations of sodium nitrite (5, 10, 25, 50, 75, and 100 μM) were prepared. The Greiss solution was added to all microplate containing sodium nitrite and supernatant and was read through an ELISA reader (stat fax100; the USA) at the wavelength of 540 nm 20.
Ferric reducing/antioxidant power (FRAP) method
FRAP method was used to measure the total antioxidant capacity of the serum. In this technique, the ability of the plasma in retaining ferric ions was measured. This process required a great quantity of FeIII. A blue stain was formed when the compound of FeIII -TPTZ in acidic pH returned to FeII and absorption at the maximum wavelength of 600 nm. The factor defining the speed of FeII -TPTZ and the blue color was the only vitalizing power of the sample. Total antioxidant capacity values are strategized by means of a standard curve with diverse concentrations of iron sulfate 21.
Statistical analysis
Kolmogorov-Smirnov test was first conducted to confirm the data compliance of the normal distribution. One-way analysis of variance (one-way ANOVA) was used for statistical analysis, and Tukey post hoc test was used to determine the difference between the groups. SPSS 16 was used for data analysis; the results were expressed as mean ± standard error, and p <0.05 was considered statistically significant.
Results
Neurons number
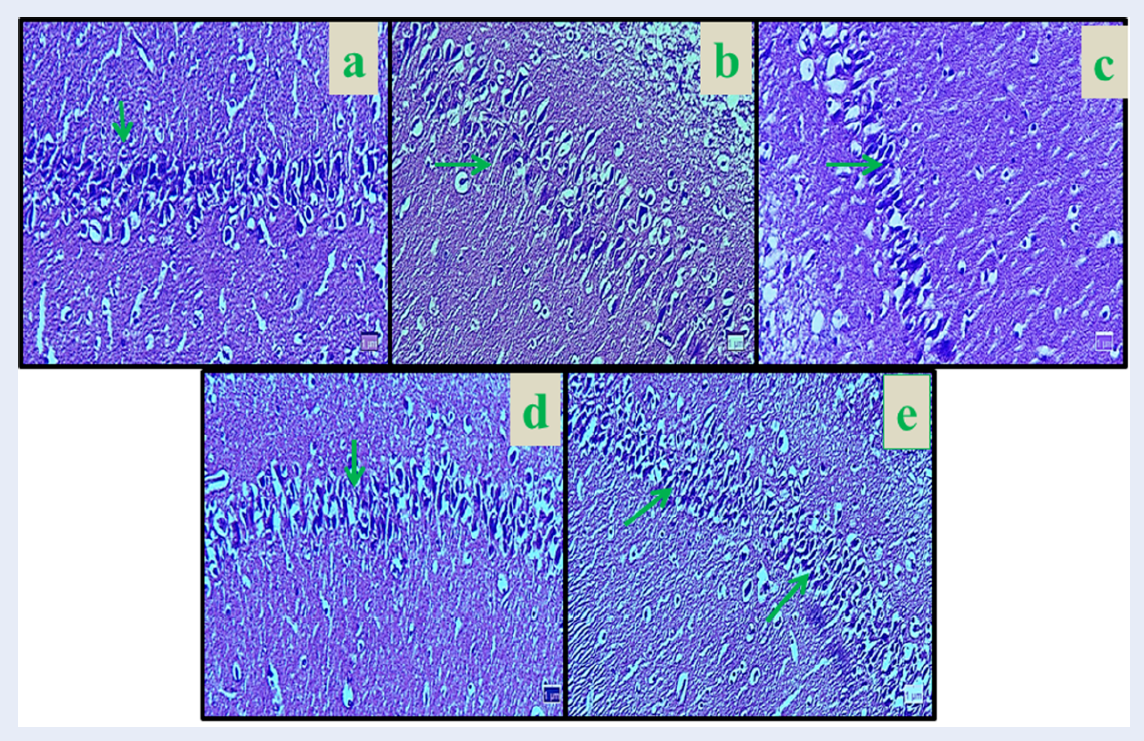
The number of neurons in the hippocampal region CA1 showed a significant decrease in Nicotine control group compared to the normal control group (8.33%) (p < 0.01). The mean number of neurons was not significantly different in all Curcumin groups compared to the normal control group (p > 0.05). The mean of pyramidal neurons increased significantly in Curcumin (dose 10 mg/kg = 18.83%, dose 30 mg/kg = 19%, and dose 60 mg/kg = 19.50%), and Nicotine + Curcumin (dose 10 mg/kg = 14%, dose 30 mg/kg = 14.66%, and dose 60 mg/kg = 14%) in all doses compared to the Nicotine control group (p < 0.01). Also, the average number of pyramidal neurons decreased significantly in all Nicotine + Curcumin groups (dose 10 mg/kg = 14.12%, dose 30 mg/kg = 15.16%, and dose 60 mg/kg = 14.61%) compared to the normal control group (p < 0.01) (Figure 1 and Figure 2).
Dendritic spines
The mean number of neuronal dendritic spines in experimental groups showed a significant decrease between normal control group and Nicotine control group (7.13%) (p < 0.01). The mean number of neuronal dendritic spines was not significant in all Curcumin groups compared to the normal control group (p > 0.05). In the Curcumin (dose 10 mg/kg = 14.85%, dose 30 mg/kg = 14.96%, and dose 60 mg/kg = 14.93%) and Nicotine + Curcumin groups (dose 10 mg/kg = 11.50%, dose 30 mg/kg = 11.66%, and dose 60 mg/kg = 11.83%), the mean number of neuronal dendritic spines increased significantly in all treated groups compared with the nicotine control group (p < 0.01). Furthermore, mean number of neuronal dendritic spines decreased significantly in all Nicotine + Curcumin groups (dose 10 mg/kg = 11.50%, dose 30 mg/kg = 11.66%, and dose 60 mg/kg = 11.83%) compared to the normal control group (p < 0.01) (Figure 3 and Figure 4).
Nitrite oxide
The results of blood serum NO measurement showed a significant increase in Nicotine control group compared to normal control group (404.285 µm) (p < 0.01). Mean nitrite oxide in the blood serum was not significant different in all Curcumin groups compared to the normal control group (p > 0.05). Also, the mean of nitrite oxide levels in blood serum declined significantly in Curcumin (dose 10 mg/kg = 190.61 µm, dose 30 mg/kg = 193.83 µm, and dose 60 mg/kg = 190.69 µm) and Nicotine + Curcumin groups (dose 10 mg/kg = 309.69µm µm, dose 30 mg/kg = 295.88µm µm, dose 60 mg/kg = 299.58µm µm) in all doses compared to the Nicotine control group (p < 0.01) (Figure 5).
Total antioxidant capacity
The results showed that total antioxidant capacity serum level reduced significantly in the Nicotine control group compared to the normal control group (p < 0.01). The total antioxidant capacity level enhanced significantly in all Curcumin (dose 10 mg/kg = 2.09 mmol/i, dose 30 mg/kg = 2.03 mmol/i, dose 60 mg/kg = 2.04 mmol/i) and nicotine + Curcumin groups (dose 10 mg/kg = 1.24 mmol/i, dose 30 mg/kg = 1.28 mmol/i, dose 60 mg/kg = 1.31 mmol/i) compared to the nicotine control group (p < 0.01) (Figure 6).
Discussion
Nicotine causes a number of implications and side effects by affecting the central and peripheral nervous system. Regarding dosage, mode of application, and species, nicotine can affect an individual’s behavior. Ventral hippocampal α4β2 blockade ‐ induced working memory deficits are reversed by chronic systemic nicotine treatment, while ventral hippocampal α7 blockade‐induced working memory deficits were not reversed by the same nicotine regimen6. Temporal lobe and hippocampal organization are involved in transferring short-term memory to long-term memory 11. The present study was aimed to investigate the effects of curcumin on nicotine-induced disorders in the hippocampus CA1 region. The results of the current study showed that the number of neurons and dendritic thorns decreased significantly in the nicotine control group compared to the normal control group. In nicotine + curcumin groups, there was a significant increase in the number of dendritic thorns compared to the nicotine control group. The results may indicate the control of apoptosis and neuro-degeneration by administering different doses of curcumin. The results of Tewari et al. were consistent with those of the present study that showed nicotine could damage the cells in the hippocampus by increased protein accumulation in the membrane and reduced cell size22. Similarly, based on the results of the current study, nicotine could decrease the number of neurons significantly in the hippocampus due to damaging the cells. It seems that nicotine induces oxidative stress and, consequently, the production of free radicals such as superoxide and hydroxyl radicals can cause cell damage 8. Generated free radicals following oxidative stress induction may have the potential to damage cellular compositions, including proteins, lipids, and DNA 20. Equally, in the current study, nicotine could decrease the number of neurons and dendritic thorns in the hippocampus due to oxidative stress caused by administration of nicotine. Exposure to nicotine can also increase the production of ROS and peroxidation of lipids, and decrease the level of GSH antioxidant enzymes 6. The brain is one of the most important organs in the body, which consumes a large amount of oxygen. Lipid in the membrane of nerve cells has a high content of oxidized unsaturated fatty acids 12. Therefore, it seems that nicotine can produce ROS via P-450 enzyme and, by producing oxidative stress, cause the destruction of the nucleus in neurons 5. Given that dendritic thorns play a major role in synaptic transmission, it is not surprising that many brain diseases are associated with changes in the morphology and density of dendritic thorns 12. Dendrite thorns are involved in memory as an exon and dendrites interface 14. Nicotine can reduce the length of dendrites and the number of dendritic thorns in nucleus accumbens by affecting neurotrophic factors in the striatum 23. The results of the study by Brown et al. showed that nicotine injections with a dose of 0.7 mg/kg could reduce the length of dendrites and the number of dendritic thorns; which is consistent with the results of our study 24. It seems that nicotine can destroy dendritic cells. Similarly, in the current study, due to oxidative stress, nicotine could decrease the number of neurons and dendritic thorns in the hippocampus horns by β2-nAChRs deactivation in postsynaptic cells in the hippocampus region 25. Moreover, nicotine can reduce the number of thorns by deactivating α4β2-nAChRs in the pre-synaptic membrane and by disrupting the release of glutamate neurotransmitters 26. It seems that nicotine reduces the number of dendritic thorns in two ways. One way is the regulation of glutamatergic synapses on pyramidal neurons. This mechanism is mainly based on the activity of α4β2 nAChRs on pre-synaptic glutamate terminus. Another mechanism focuses on the activity of GABA in the internal neurons or interneurons 27. Curcumin is a neutralizer of reactive oxygen species ROS, which has the potential to destroy oxidative stress and prevents lipid peroxidation6. The results of the study by Shin et al. confirmed those of the present study that curcumin could prevent cell death from kainic acid due to oxidative stress in the hippocampus 28. Curcumin seems to inhibit lipid peroxidation of quinolinic acid and controls the production of cyanide-induced superoxide in the brain, suggesting the protective properties of curcumin. In addition, nicotine treatment increased lipid peroxidation and the levels of GSH, IL-1β, TNF-α, and Bax, while reducing Bcl-2, P-CREB, and BDNF levels in the hippocampus 29. Curcumin has been shown to significantly improve spatial memory impairment induced by HIV-1 gp120 V3 in rats, but the electrophysiological mechanism remains unknown 30. The results of a study by Pan et al. are in line with the results of the present study, which showed that curcumin improves learning and memory in mice and can increase neuroprotective effects in Alzheimer’s modal rats 31. Curcumin can reduce neuropathological changes in the hippocampus and control apoptosis by increasing the density of Bcl-2 protein 32. The present study showed a significant increase in serum NO levels in the nicotine control group compared to the normal control group. In all nicotine + curcumin groups, there was a significant decrease in serum NO levels in comparison to the nicotine control group. NO is a free radical, and that can regulate angiogenesis, apoptosis, cell cycle, invasion, and metastasis in cells. NO plays a key role in the destruction of myelin in the central nervous system11. Nicotine can stimulate nicotinic receptors in the brain and increase glutamate release and NMDA activation. The activation of NMDA may increase the formation of nitric oxide in the hippocampus 33. The results of a study by Keser et al. revealed that the exposure to nicotine increased the activity of NO in the frontal cortex in the mouse brain. This result confirms the results of the present study 34. Also, the results of Salahshoor et al. are consistent with those of the present study, which shows that curcumin prescription significantly decreased the serum level of NO in the blood serum of mice 8. The results of this study showed that there was a significant decrease in total antioxidant levels in the nicotine control group compared to the normal control group. In all nicotine + curcumin groups, there was a significant decrease in serum total antioxidant levels in comparison to the nicotine control group. The results of the study by Zhuo et al. showed that total antioxidant level was reduced significantly in smokers in proportion to non-smokers; which is consistent with the results of a similar study 35. It seems that the reduction of total antioxidant capacity in plasma of smokers has led to an increase in the production of free radicals 36. The reduction in total antioxidant capacity levels in this study shows the effects of oxidative stress from nicotine on the hippocampal neurons. This result is expressed as growth in the levels of ROS, lipid peroxidation, and a reduction in the action of antioxidant enzymes such as total antioxidant capacity 37. Curcumin, via activation of P-CREB/BDNF signaling pathway, confers neuroprotection against nicotine-induced inflammation, apoptosis, and oxidative stress. In contrast, various doses of curcumin attenuated nicotine-induced apoptosis, oxidative stress, and inflammation, while elevating P-CREB and BDNF levels. Thus, curcumin, via activation of P-CREB/BDNF signaling pathway, confers neuroprotection against nicotine-induced inflammation, apoptosis, and oxidative stress 38. In the present study, improved levels of total antioxidant capacity in rats treated with curcumin highlighted the antioxidant and anti-lipid peroxidation effects of curcumin. An increase in total antioxidant levels due to the administration of nicotine in the present work may suggest the positive impact of curcumin on magnified antioxidant effects, the inhibition of nicotine-induced inflammation and the destruction process on neurons in the brain. Curcumin can inhibit nitric oxide by reducing the activity of the nuclear factor-kappa β (NF-Kβ) 39. Our study had certain limitations, such as the lack of detecting antioxidant levels in the curcumin. There was a lack of references about the effect of plant or extract in CA1 region, and death of some animals due to nicotine administration in this study was an important limitation. Hence, prospective studies should be taken for the detailed association of the molecular interaction between Curcumin and CA1 region in rats.
Conclusion
The current study indicated that curcumin could recover some CA1 injuries significantly, which is contrary to the destructive properties of nicotine in rats. It appears that curcumin provides protection against oxidative stress resulting from nicotine. Such an ability of curcumin might be due to its strong potential antioxidant attributes. As a result, it leads to CA1 tissue recovery and prevention of nicotine adverse effects on total antioxidant capacity, nitric oxide, number of neurons, and dendritic spines, as evidenced in the above mentioned examination of the male rats. However, supplementary studies are essential to describe its molecular mechanism.
Abbreviations
CA1: Cornu Ammonis 1
DNA: Deoxyribonucleic acid
FRAP: Ferric reducing/antioxidant power
GABA: Gamma-Aminobutyric Acid
nAChRs: nicotinic acetylcholine receptors
NO: Nitrite oxide
ROS: Reactive oxygen species
Author Contributions
CJ conceived and designed the experiments, CJ and SMR revised the manuscript, SR and ARB analyzed the data and MRS wrote the paper. All authors read and confirmed the publication of the article.
Financial support and sponsorship
We are grateful to the Research Council of Kermanshah University of Medical Sciences (No: 1396.562) for their financial support.
Competing interests
The authors declare they have no competing interests.
References
-
Sharapova
S R,
Singh
T,
Agaku
I T,
Kennedy
S M,
King
B A,
Patterns of E-cigarette Use Frequency-National Adult Tobacco Survey. American Journal of Preventive Medicine.
2018;
54
:
284-288
.
View Article PubMed Google Scholar -
Pacek
L R,
Oliver
J A,
Sweitzer
M M,
Mcclernon
F J,
Young adult dual combusted cigarette and e-cigarette users anticipated responses to a nicotine reduction policy and menthol ban in combusted cigarettes. Drug alcohol dependence.
2019;
194
:
40-44
.
View Article PubMed Google Scholar -
Filbey
F M,
Gohel
S,
Prashad
S,
Biswal
B B,
Differential associations of combined vs. isolated cannabis and nicotine on brain resting state networks. Brain Structure and Function.
2018;
7
:
3317-3326
.
-
Kimura
I,
Dohgu
S,
Takata
F,
Matsumoto
J,
Kawahara
Y,
Nishihira
M,
Sakada
S,
Saisho
T,
Yamauchi
A,
Kataoka
Y,
Activation of the α7 nicotinic acetylcholine receptor upregulates blood-brain barrier function through increased claudin-5 and occludin expression in rat brain endothelial cells. Neuroscience Letters.
2019;
694
:
9-13
.
View Article PubMed Google Scholar -
Jalili
C,
Ahmadi
S,
Roshankhah
S,
Salahshoor
M,
Effect of genistein on reproductive parameter and serum nitric oxide levels in morphine‑treated mice. International Journal of Reproductive Biomedicine (Yazd).
2016;
14
:
95-102
.
View Article PubMed Google Scholar -
Jalili
C,
Salahshoor
M R,
Khademi
F,
Jalili
P,
Roshankhah
S H,
Morphometrical Analysis of the Effect of Nicotine Administration on Brains Prefrontal Region in Male Rat. International Journal of Morphology.
2014;
32
:
761-766
.
View Article Google Scholar -
Fricker
M,
Goggins
B J,
Mateer
S,
Jones
B,
Kim
R Y,
Gellatly
S L,
Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI insight.
2018;
3
:
94040
.
View Article PubMed Google Scholar -
Salahshoor
M R,
Mohamadian
S,
Kakabaraei
S,
Roshankhah
Sh,
Jalili
C,
Curcumin Improves liver Damage in Male Mice Exposed to Nicotine. Journal of Traditional and Complementary Medicine.
2016;
6
:
176-183
.
View Article PubMed Google Scholar -
Kuo
Y H,
Huang
T T,
Chou
E Y,
Chiang
Y C,
Hoffer
Y H,
Miller
B J,
.
J,
Effect of traumatic brain injury on nicotine-induced modulation of dopamine release in the striatum and nucleus accumbens shell. Oncotarget.
2018;
9
:
10016-10028
.
View Article PubMed Google Scholar -
Changeux
J P,
Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nature Reviews Neuroscience.
2010;
11
:
389-401
.
View Article PubMed Google Scholar -
Jalili
C,
Salahshoor
M R,
Pourmotabbed
A,
Moradi
S,
Motaghi
M,
Darehdori
A Shabanizadeh,
The Effects of Aqueous Extract of Boswellia Serrata on Hippocampal Region CA1 and learning deficit in kindled Rats. Research in pharmaceutical science.
2014;
9
:
351-358
.
PubMed Google Scholar -
Moradi
S,
Pourmotabbed
A,
Salahshoor
M R,
Jalili
C,
Motaghi
M,
Kakebaraei
S,
The Morphometric Effects of Aqueous Extract of Boswellia Serrata on Hippocampal Region CA1 in kindled Rats. Int J Morphol.
2014;
32
:
1271-1276
.
-
Wink
M,
Modes of action of herbal medicines and plant secondary metabolites. Medicines.
2015;
2
:
251-286
.
View Article PubMed Google Scholar -
Oyarce
P,
De
-,
Meester
B,
Fonseca
F,
De
-,
Vries
L,
Goeminne
G,
Pallidis
A,
Introducing curcumin biosynthesis in Arabidopsis enhances lignocellulosic biomass processing. Nature Plants.
2019;
5
:
225-237
.
View Article PubMed Google Scholar -
Jalili
C,
Salahshoor
M R,
Jalili
F,
Kakaberaei
S,
Akrami
A,
Sohrabi
M,
Therapeutic effect of resveratrol on morphine‑induced damage in male reproductive system of mice by reducing nitric oxide serum level. International Journal of Morphology.
2017;
35
:
1342-1347
.
View Article Google Scholar -
Jayaprakasha
G K,
Jagan
-,
Mohan
-,
Rao
L,
Sakariah
K K,
Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Journal of agricultural and food chemistry.
2002;
50
:
3668-3672
.
View Article PubMed Google Scholar -
Belviranl
M,
Okudan
N,
Atalk
K E N,
Öz
M,
Curcumin improves spatial memory and decreases oxidative damage in aged female rats. Biogerontology.
2013;
14
:
187-196
.
View Article PubMed Google Scholar -
Antosiak
A,
Milowska
K,
Maczynska
K,
Rozalska
S,
Gabryelak
T,
Cytotoxic activity of genistein‑8‑C‑glucoside form Lupinus luteus L. and genistein against human SK‑OV‑3 ovarian carcinoma cell line. Medicinal Chemistry Research.
2017;
26
:
64-73
.
View Article PubMed Google Scholar -
Jalili
C,
Salahshoor
M R,
Khani
F,
Roshankhah
S H,
Protective Effect of Curcumin against Nicotine-induced Damage on Reproductive Parameters in Male Mice. International Journal of Morphology.
2014;
32
:
869-874
.
View Article Google Scholar -
Salahshoor
M R,
Roshankhah
S,
Hosseni
P,
Jalili
C,
Genistein Improves Liver Damage in Male Mice Exposed to Morphine. Chinese Medical Journa.
2018;
131
:
1598-1604
.
View Article PubMed Google Scholar -
Ghorbani
R,
Mokhtari
T,
Khazaei
M,
Salahshoor
M R,
Jalili
C,
Bakhtiari
M,
The effect of walnut on the weight, blood glucose and sex hormones of diabetic male rats. International Journal of Morphology.
2014;
32
:
858-8209
.
View Article Google Scholar -
Tewari
A,
Misra
R,
Neurochemical Effects of Nicotine on Albino Rats. Brain. Arch Neurosci.
2014;
1
:
51-54
.
View Article Google Scholar -
Androuin
A,
Potier
B,
Ngerl
U V,
Cattaert
D,
Danglot
L,
Thierry
M,
Evidence for altered dendritic spine compartmentalization in Alzheimers disease and functional effects in a mouse model. Acta Neuropathologica.
2018;
25
:
1-6
.
View Article PubMed Google Scholar -
Brown
R,
Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Research.
2001;
899
:
94-100
.
View Article PubMed Google Scholar -
Eftekhari
A,
Ahmadian
E,
Panahi‑Azar
V,
Hosseini
H,
Tabibiazar
M,
Dizaj
S Maleki,,
Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1‑induced liver damage: In vitro/in vivo studies. Artificial Cells, Nanomedicine, and Biotechnology.
2018;
46
:
420-420
.
View Article PubMed Google Scholar -
Oda
A,
Nakagomi
S,
Nicotine induces dendritic spine remodeling in cultured hippocampel neurons. J Neurochemistry.
2014;
128
:
246-255
.
View Article PubMed Google Scholar -
Zambrano
C A,
Escobar
D,
Ramos-Santiago
T,
Bollinger
I,
Stitzel
J,
Serine residues in the α4 nicotinic acetylcholine receptor subunit regulate surface α4β2* receptor expression and clustering. Biochemichal Pharmacology.
2019;
159
:
64-73
.
View Article PubMed Google Scholar -
Shin
H J,
Son
E,
Lee
D H,
Kim
H J,
Kang
S S,
Cho
G J,
Choi
W S,
Roh
G,
Curcumin attenuate the Kainic acid Induced hippocampal cell death in mice. Neuroscience letters.
2007;
416
:
49-54
.
View Article PubMed Google Scholar -
Bartos
M,
Gumilar
F,
Gallegos
C E,
Bras
C,
Dominguez
S,
Mnaco
N,
Alterations in the memory of rat offspring exposed to low levels of fluoride during gestation and lactation: Involvement of the 7 nicotinic receptor and oxidative stress. Reproductive Toxicology.
2018;
81
:
108-114
.
View Article PubMed Google Scholar -
Shen
L L,
Jiang
M L,
Liu
S S,
Cai
M C,
Hong
Z Q,
Lin
L Q,
Curcumin improves synaptic plasticity impairment induced by HIV-1gp120 V3 loop. Neural Regeneration Research.
2015;
10
:
925-931
.
View Article PubMed Google Scholar -
Pan
R,
Lu
D X,
Dong
J,
Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chinese Medical Journal.
2008;
121
:
832-839
.
View Article PubMed Google Scholar -
Muthuraman
A,
Thilagavathi
L,
Jabeen
S,
Ravishankar
S B,
Ahmed
S S,
George
T,
Curcumin prevents cigarette smoke extract induced cognitive impairment. Frontiers in Bioscience.
2019;
11
:
109-120
.
View Article Google Scholar -
Piri
M,
Nasehi
M,
Shahab
Z,
Zarrindast
M R,
The effects of nicotine on nitric oxide induced anxiogenic-like behaviors in the dorsal hippocampus. Neuroscience Letters.
2012;
528
:
93-98
.
View Article PubMed Google Scholar -
Keser
A,
Nesil
T,
Kanit
L,
Pogun
S,
Brain nitric oxide metabolites in rats preselected for nicotine preference and intake. Neuroscience Letters.
2013;
545
:
102-106
.
View Article PubMed Google Scholar -
Zhuo
L,
Liao
M,
Zheng
L,
He
M,
Huang
Q,
Wei
L,
Combination therapy with taurine, epigallocatechin gallate and genistein for protection against hepatic fibrosis induced by alcohol in rats. Biological and Pharmaceutical Bulletin.
2012;
35
:
1802-1810
.
View Article PubMed Google Scholar -
Isik
B,
Ceylan
A,
Isik
R,
Oxidative stress in smokers and non-smokers. Inhalation Toxicology.
2007;
19
:
767-769
.
View Article PubMed Google Scholar -
Schreiber
R,
Buchholz
B,
Kraus
A,
Schley
G,
Scholz
J,
Ousingsawat
J,
Lipid Peroxidation Drives Renal Cyst Growth In Vitro through Activation of TMEM16A. Journal of American Society of Nephrology.
2019;
30
:
228-242
.
View Article PubMed Google Scholar -
Motaghinejad
M,
Motevalian
M,
Fatima
S,
Faraji
F,
Mozaffari
S,
The Neuroprotective Effect of Curcumin Against Nicotine-Induced Neurotoxicity is Mediated by CREB-BDNF Signaling Pathway. Neurochemichal research.
2017;
42
:
2921-2932
.
View Article Google Scholar -
Akaishi
T,
Abe
K,
cnb-001, a synthetic pyrazole derivative of curcumin, suppresses lipopolysaccharide-induced nitric oxide production through the inhibition of Nf-b and p38 Mapk pathways in microglia. European Journal of Pharmacology.
2018;
819
:
190-197
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 6 No 9 (2019)
Page No.: 3368-3378
Published on: 2019-09-23
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4520 times
- View Article downloaded - 0 times
- Download PDF downloaded - 911 times
 Biomedpress
Biomedpress







