Abstract
Background: Antimicrobial Stewardship (AMS) programs are increasingly implemented in healthcare facilities to reduce inappropriate use of antibiotics. Inappropriate use of antimicrobials is one of the leading causes of increasing antimicrobial resistance (AMR). As such, decreasing inappropriate use of antimicrobials can reduce AMR. The main objective of this systematic review was to identify the effectiveness of AMS interventions in reducing inappropriate antimicrobial use in residential aged care facilities (RACFs).
Methods: Multiple databases were searched to identify studies evaluating the effectiveness of AMS interventions in RACFs. Descriptive studies and those, which did not report an outcome measure related to antibiotic usage, were excluded.
Results: Overall, 6505 studies were identified and 17 were included. Most of the studies were randomized controlled trials and single faceted interventions. All of the interventions were education and training targeted at physicians, nurses or both about appropriate use of antimicrobials in RACFs. The studies reported a high success rate with 68% of interventions being successful while 32% of interventions were found to be ineffective.
Conclusion: Educating and training nurses and physicians about evidence-based management of infectious diseases may lead to a reduction in inappropriate antimicrobial use in RACFs. The likelihood of success increases with targeting physicians - either as the sole recipient of the intervention or together- with nurses.
Introduction
The World Health Organization (WHO) declared antimicrobial resistance (AMR) as one of the threats to human health with an estimated mortality amounting to 10 million deaths annually by 20502, 1. The causes of AMR are multifactorial. Inappropriate use of antimicrobials is one of the modifiable factors responsible for increasing AMR. The correlation between AMR and inappropriate antimicrobial use has been reported by numerous studies6, 5, 4, 3. Moreover, more than half of the antimicrobials used in residential aged care facilities (RACFs) were inappropriate, according to different international studies10, 9, 8, 7. Worldwide, it is estimated that 700,000 deaths every year are due to AMR11. Decreasing inappropriate use of antimicrobials can reduce AMR.
A number of factors are responsible for the significant increase in AMR including a lack of commercial production of new antimicrobials, wide use of antimicrobials in agriculture, and inappropriate or over usage of antimicrobials11. Approximately, 1.5 billion euros are spent annually in Europe12 and $200 million in Canada due to AMR13. The government of the United Kingdom published a 5-year Antimicrobial Resistance Strategy (2013-2018) to overcome the burden of AMR14.
Due to high usage of antibiotics, antimicrobial-resistant organisms are highly prevalent in RACFs16, 15. There are about 1.4 million people living in RACFs in the United States of America (USA)17, 2 million in Canada, and 1.24 million in the UK18. Approximately, 1.6 to 3 million cases of infections were diagnosed every year in the US nursing homes19. Many infections become difficult to diagnose and treat, which is a major cause of mortality and morbidity in nursing homes. This difficulty is mainly due to development of resistance or changes in the causative agents of infections20. The most common infections in RACFs are lower respiratory tract infections (LRTIs), urinary tract infections (UTIs), skin and soft tissues infections (SSTIs), and gastroenteritis (GE)19. There is a 40-70% chance of exposure to at least one course of antibiotics if a resident resides in RACFs for 6 months22, 21.
Some studies reported that about half of the antibiotics used in RACFs are potentially inappropriate or unnecessary27, 26, 25, 24, 23, 22. The inappropriateness is mainly linked with the adequacy of empiric antibiotic choice, wrong dose, and prolonged duration of antibiotic use29, 28. In the EU countries, it is estimated that 57% of antibiotics are used in upper respiratory tract infections, followed by 30% in lower respiratory tract infections and 7% in urinary tract infections30. In many RACFs, the decision on prescribing antibiotics is made off-site by telephone, depending on limited laboratory and clinical information, and therefore, highly influenced by staff members of these facilities33, 32, 31. Pressure from nursing home staff and family members leads to unnecessary prescribing of antibiotics in the American, Canadian and Australian RACFs34, 33, 32, 31. Responding to the widespread over-use and inappropriate use of antimicrobials, several countries have introduced quality initiatives in a bid to reduce AMR36, 35.
Antimicrobial Stewardship (AMS) is a collective term used to make strategies to reduce the inappropriateness of antimicrobial use and minimize the adverse effects of the antimicrobials, which include toxicity, cost, and resistance37. AMS programs have been recommended all over the world for the better prevention and management of infections in RACFs38. A range of interventions has been developed to improve antibiotic use in RACFs; however, there is a limited understanding of the outcomes of those interventions. There is uncertainty among healthcare providers concerning optimal approaches to improve antibiotic use in RACFs39. To the best of our knowledge, there is no systematic review investigating AMS interventions in RACFs. The aim of this systemic review was to systematically identify and evaluate the effectiveness of AMS interventions in RACFs.
Methods
Search strategy and data source
We searched CINAHL, Pubmed, Cochrane and Embase databases from inception through February 2018. A combination of Medical Subject Heading (MeSH) terms and keywords like “home for the aged”, “nursing home”, “residential care”, “long-term care” and “infections” were used as search terms. Search details are provided in the appendix. To identify any additional relevant publications, a cross-reference search from included studies was also conducted.
The guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Figure 2 were followed for this systematic review40. The PRISMA checklist is attached in the appendix.
Selection of studies
Titles and abstracts were screened for the identification of relevant studies. Interventions related to infections in RACFs were included in this review. Potential articles were assessed independently for inclusion (AA and SF) and any disagreement was resolved by discussion with a third reviewer (AHK).
Studies published in English in peer-reviewed journals investigating infection-related interventions conducted in RACFs were included (Figure 1).
Data extraction and analysis
One reviewer (AA) extracted relevant data from included studies with the help of Endnote software, and this was verified by a second reviewer (SF). All the extracted information was saved in the Endnote library. Extracted information from the selected studies included author, publication year, study design and setting, country of origin, type of interventions, and the outcomes and findings based on the interventions (Table 1).
| Reference, year, country | Study Design | Interventions | Outcomes | Findings |
|---|---|---|---|---|
| Education + active surveillance/ audit and feed back | ||||
| Mody et al., 201555 USA | Randomized clinical trial, 12 community-based NHs | NH staff education and active surveillance of resistant infections (MDRO). | Prevalence of MDRO and MRSA | Significant reduction in prevalence of MDRO [(23%) (rate ratio, 0.77; 95%CI, 0.62-0.94; p = 0.01)] and MRSA [(22%) rate ratio, 0.78; 95% CI, 0.64-0.96; p = 0.01] |
| Zimmerman et al.50, 2014 USA | Quasi-experimental trial, 12 NHs | Educational sessions for physicians, nurses and families of residents along with feedback. | Antibiotic prescribing rates for different infections. | Antibiotic prescription decrease by 11.1 prescription per 1000 residents-days and 1.8 prescriptions avoided per 1000 resident-days. |
| Pettersson et al.51, 2011 Sweden | Cluster randomized controlled trial, 58 NHs | Education on antibiotic prescribing with educational materials, audit and feedback. | Prescription of quinolones for lower UTIs in women. | Significant decrease in quinolones prescription in IG -0.196 (95% CI, -0.338, -0.054) and CG -0.224 (95% CI -0.394, -0.054). |
| Baldwin et al.48, 2010 UK | Cluster randomised controlled trial, 32 NHs | Infection control training, demonstration on hand hygiene, audit and feedback. | Prevalence of MRSA among residents and staff, along with audit. | Prevalence of MRSA was non-significant however, mean infection audit score was increased (82%) (p < 0.0001) at 12 months. |
| Monette et al.46, 2007 Canada | Cluster randomized, controlled trial, 8 LTC facilities | Mailing of antibiotic guide along with previous 3 months antibiotic prescribing pattern to 36 physicians twice with 4 months difference. | Adherence to antibiotic prescribing guidelines. | Physicians were 64% less likely to prescribe non-adherent antibiotics in IG (OR = 0.36, 95% CI, 0.18-0.73). |
| Implemented Educational program | ||||
| Van Gaal et al.41, 2010 Netherlands | Cluster randomised trial, 10 Hospital wards and 10 nursing home wards | The educational material consisted of an educational compact disc for nurses. | The test score of nurses for pressure ulcers and UTIs. | Non-significant UTIs knowledge was observed [0.17 points (95% CI; -0.31 to 0.65)] however; knowledge on pressure ulcers was statistically significant [0.45 points (95% CI; 0.10-0.81)]. |
| Zabarsky et al. 56,2008 USA | Before/ After, Single LTCF | Education to physicians about diagnosis and treatment of ASB and nurses about the urine cultures for UTIs. | Treatment of ASB and appropriateness of urine cultures. | Inappropriate treatment reduces from 1.7 to 0.6 per 1000 patient days (p = 0.0017) and urine cultures decreased from 2.6 to 0.9/1000 patient days (p < 0.0001). |
| Hasson et al.52 , 2006 Sweden | Prospective, non-randomized, controlled intervention, 2 elderly care organizations | 16 item educational tool for NH staff and residents families for management of infections. | The effect of the educational intervention on NH staff, residents and their families to improve the quality of care. | There were no significant changes in quality of care as perceived by residents or their families. |
| Education along with dedicated support | ||||
| Van Buul et al. 53, 2015 Netherlands | Quasi-experimental, before/after, 10 NHs | Improve physician knowledge, physician-nursing staff communication, Optimizing medication formularies and utilization of diagnostic resources. | Appropriate decisions regarding prescribing or withholding antibiotics for every infection. | No pre- post- test difference in appropriate prescribing decisions in the intervention group (82% to 79%; p = 0.28). |
| Jump et al. 57,2012 USA | Before/After, 4 LTCFs | LID consult team along with 24 hours telephone support. | Total use of antimicrobials in LTCFs. | Reduction in the use of antimicrobials by 30.1% (p < 0.001). |
| Daly et al. 42, 1992 USA | Before/After, 3 NHs | Two days infection control training along with telephone support. | To evaluate the knowledge and practices of NH staff regarding management of infections. | Knowledge: after training overall a 25% increase in correctly answered questions (F = 1024; df = 1/263; p < 0.0001). Significant time spent on infection control (F = 37; df = 1/209; p < 0.0001) after training than before training.Significant increase in practices for all training sites (F = 139.5; df = 1/209, p < 0.0001). |
| Linnebur et al. 43, 2011 USA | Quasi-experimental study, 16 NHs | Academic detailing to physicians by pharmacists and educational sessions for nurses regarding diagnostic and prescribing practices of NHAP. | Adherence to antibiotic guidelines. | Antibiotic guidelines adherence increases from 60% to 66% (p = 0.3). |
| Development and Implementation of local guidelines | ||||
| Rummukainen et al. 54,2012 Finland | Before/After39 NH | Development of guidelines for diagnostic practices for UTIs and educating NH staff about them. | Antibiotic prophylaxis used for UTIs. | Decreased the use of antibiotics for UTIs from 13% to 6%. |
| Schwartz et al.44, 2007 USA | Quasi-experimental before–after | Teaching sessions for physicians about guidelines for management of infections. | Management of infections along with treatment based on the guidelines. | Charted clinical abnormalities met guideline diagnostic criteria: 62% vs 38% (p = 0.006) and treatment based on guidelines: 39% vs 11% (p < 0.001). |
| Naughton et al.45, 2001 USA | Randomized controlled trial, 10 Skilled nursing facilities | NHAP guidelines development and educating physicians and nurses (pocket booklets). | Antibiotic adherence. | No significant difference was found between pre and post intervention groups (p = 0.86). |
| Decision Support Tools | ||||
| Fleet et al. 49, 2014 UK | Cluster randomized control, 30 NHs | Introduction of Resident Antimicrobial Management Plan (RAMP) antimicrobial stewardship tool completed by nursing staff for all residents. | Change in use of systemic antibiotics for the treatment of infections. | Significantly decreased in prescription rate by 4.9% (95% CI 1.0% to 8.6%; p = 0.02). |
| Loeb et al.47, 2005 Canada and USA | Cluster randomised controlled trial, 24 NHs | Specific algorithms for physicians and nurses regarding UTIs, individual interviews with physicians. | Antimicrobials prescribed for suspected UTIs, antimicrobials leading to hospital admissions or death. | 1.The rate of antimicrobial use for suspected urinary tract infection was significantly lower in the intervention arm than in usual care arm (1.17 courses of antimicrobials per 1000 resident days prescribed vs 1.59; weighted mean difference -0.49, 95% CI -0.93 to -0.06).2. No significant difference was found in admissions to hospital or mortality between the study arms. |
Quality assessment
Two reviewers (AA and SF) independently carried out the quality assessment of included studies by using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool58 (Appendix). This tool evaluates six quality-related domains like study design, selection bias, blinding, confounders, withdrawals and dropouts, and data collection methods. Any disagreement was resolved by discussion with a third reviewer (AHK).
Results
Description of included studies
Seventeen articles met the inclusion criteria (Figure 1). Table 1 summarizes the study characteristics. Most studies were conducted in North America with 8 in the USA45, 52, 51, 50, 47, 42, 1 in Canada57, and 1 as a multicenter study in the USA and Canada44. The remaining 7 studies were conducted in Europe with 2 in the United Kingdom46, 56, 2 each in the Netherlands48, 43 and Sweden53, 49, and 1 in Finland58.
Most of the studies (n = 10) were randomized controlled trials (RCTs) and 7 studies were quasi-experimental trials. Two out of 10 RCTs were standard RCTs while 8 were cluster RCTs (c-RCTs). Most quasi-experimental trails (n=7) were before-and-after study design. The number of included RACFs varied greatly from 2 RACFs to 58 RACFs (Table 1).
Types of interventions
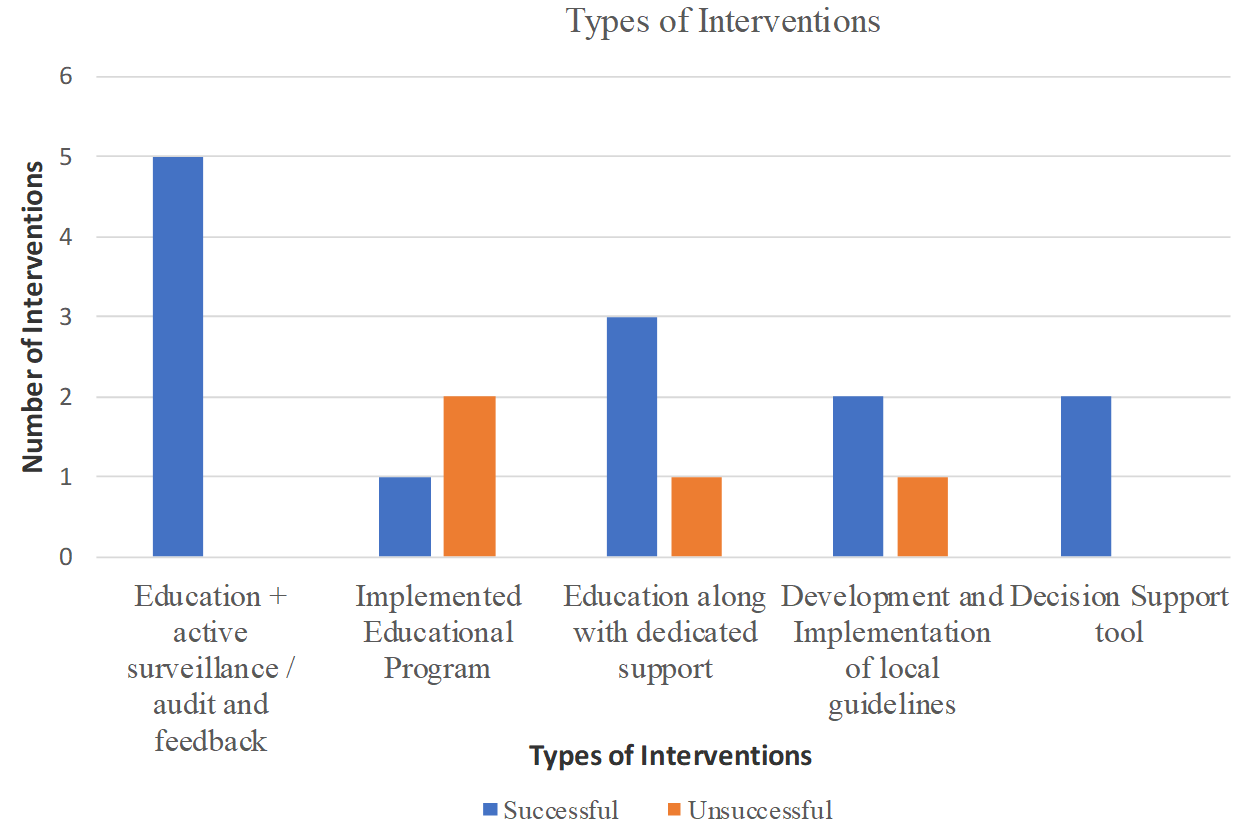
Most studies used educational interventions (70.6 %, n = 12), of which 4 studies used an educational component along with a support (through dedicated personnel), 5 studies used an educational component in addition to an active surveillance of resistant infections and/or audit and feedback, while 3 studies implemented an education only program. The remaining studies employed interventions related to the development and implementation of local guidelines (17.7 %, n = 3) and the use of decision support tools (11.7 %, n = 2) (Table 1).
Educational interventions
Three studies53, 43, 50 that implemented education only interventions introduced an educational compact disc (CD)43, a 16-item instrument53 for nursing staff to improve the knowledge regarding infections, and an educational support to physicians and nurses about the diagnosis and treatment of asymptomatic bacteriuria (ASB) and the urine cultures for UTIs, respectively. The first two interventions did not provide a statistically significant increase in knowledge of nursing staff, but the third intervention reduced inappropriate treatment of ASB from 167.1 to 117.4/1000 patient days (p<0.001) and the number of urine cultures decreased from 2.6 to 0.9/1000 patient days (p<0.0001).
Four studies included educational interventions along with dedicated personnel support48, 54, 52, 51. One of the interventions was to improve the communication of the physicians and nurses through discussion meetings regarding the management of infections in RACFs48. Medication formularies were optimized along with correct utilization of diagnostic tools to support decisions whether or not to prescribe or withhold antibiotics for any infection. There was no pre-post test difference in appropriate prescribing decisions in the intervention group (IG) (82% to 79%; p = 0.28). Moreover, appropriateness in the control group (CG) was also not increased (70% to 77%; p = 0.06). One study investigated the effect of two-day infection control training on nursing staff to evaluate their knowledge and practices about the management of infections in RACFs52. This intervention increased the knowledge of nursing staff by 25% (F =1024; df =1/263; p<0.0001) and significantly improved nurses’ practices regarding the treatment of infections (F =139.5; df = 1/209, p<0.0001). In a study investigating the effects of an infectious disease control team with 24 hr telephone support to improve the appropriate use of antimicrobials, post-intervention inappropriate use was reduced by 30.1% (p<0.001)51. In another study, pharmacists gave academic detailing to physicians and provided educational sessions to nurses to improve their adherence to nursing home-acquired pneumonia (NHAP) guidelines54. Adherence score for optimal antibiotic use increased from 60% to 66% but it was not statistically significant (p = 0.3) and adherence to guidelines regarding the use of antibiotics within the first 4 hours of NHAP diagnosis increased from 57% to 75% (p<0.001).
Five studies involved educational interventions along with active surveillance and audit/feedback for better management of infections in RACFs. In one study, mailing of an antibiotic guide with the past 3-month prescribing patterns improved the use of antibiotics. Post-intervention findings revealed that physicians were 64% less likely to prescribe non-adherent antibiotics in the IG (OR = 0.36, 95% CI, 0.18-0.73)57. In another study, educational materials including leaflets and handouts for physicians, followed by audit and feedback, resulted in a significant decrease in quinolone prescription in IG -0.196 (95% CI, -0.338, -0.054) and CG -0.224 (95% CI -0.394, -0.054)49. Two studies investigated the effect of education on nursing staff about infections caused by multi-drug resistant organisms (MDRO) and methicillin-resistant Staphylococcus aureus (MRSA)56, 42. One study42 conducted active surveillance for MDRO related infections while other another study56 conducted infection control audits. MDRO prevalence decreased by 23% in the IG (rate ratio RR 0.77; 95% CI, 0.62-0.94)42, while there was no significant difference in MRSA infections56. The final intervention included educational training for physicians and nurses regarding antibiotic-prescribing guidelines. Feedback on antibiotic prescribing was also shared with the healthcare professionals. This intervention helped to reduce prescriptions for antibiotics in RACFs [IRR 0.86 (95% CI 0.79 - 0.90)].
Development and implementation of local guidelines
Three out of 17 studies developed and implemented guidelines for the prevention and management of infections in RACFs59, 58, 45. Two studies developed and implemented guidelines to improve antimicrobial prescribing in nursing homes for infectious diseases, including NHAP and UTIs58, 45. Adherence to NHAP guidelines increased significantly in the post-intervention group (p<0.02)45, while the use of antibiotics decreased from 13% to 6% (p<0.001) in the case of UTIs58. In one study, teaching sessions were conducted for physicians on the guidelines for the management of infections. These sessions met guideline diagnostic criteria: 62% vs 38% (p = 0.006), and treatment based on guidelines: 39% vs 11% (p<0.001)59.
Decision support tools
One study introduced a Resident Antimicrobial Management Plan (RAMP) to improve the use of antibiotics in nursing homes. The purpose of RAMP was to document the prescribing, administration, and monitoring of antimicrobials46. The RAMP decreased 4.9% (95% CI: 1.0% - 8.6%) of the antibiotic prescription in the intervention group as compared to the control group. Another study introduced diagnostic and therapeutic algorithms for UTIs which resulted in a significant decrease in the use of antimicrobials in the intervention arm than in usual care arm (1.17 courses of antimicrobials per 1000 resident days prescribed vs 1.59; weighted mean difference -0.49, 95% CI -0.93 to -0.06)44.
Features of successful interventions
Figure 1 shows a breakdown of interventions and their relative successfulness in improving the use of antibiotics in RACFs. Of all educational interventions (n=12), 9 interventions (64.3%) were successful in improving the antibiotic use. However, it is noteworthy to mention that educational interventions that were combined with other interactive active surveillance/audit/feedback (80%) and personnel support (66.7%) were more effective than education only interventions (33.3%). Other interventions, such as development and implementation of guidelines (n=2) and the use of decision support tools (n=2), were all successful in improving antimicrobial use. Studies that used quasi-experimental design implemented more interventions successfully (75%) than studies using randomized control trial (70%).
Quality Assessment
According to the Effective Public Health Practice Project (EPHPP) quality assessment tool, most of the studies (n = 13) were rated as moderate quality (Appendix). The reason behind this is the information lacking about the blinding of the studies48, 43, 46, 56, 44, 45, 52, 51. The remaining four studies were rated as strong quality since the information in these studies was very clear and comprehensive 57, 55, 42, 41.
Discussion
Importance of this review
Advances in healthcare mean that the relative proportion of people living in RACFs is likely to increase over the considerable foreseeable future. Emerging reports of increasing antimicrobial resistance in RACFs is a growing concern amongst the residents, healthcare professionals, and managers of RACFs. Not surprisingly, there has been an increasing interest in the development and implementation of AMS initiatives in the RACFs worldwide. Since there was a paucity of information regarding the successful interventions to improve antimicrobial use in RACFs, this systematic review was conducted to provide an overview of different infection-related interventions in RACFs all over the world.
Principal findings
This systematic review found seventeen studies that assessed the interventions to improve antimicrobial use in RACFs. The educational interventions combined with other strategies and the multifaceted interventions mostly showed consistent and positive effects in improving the use of antimicrobial in RACFs.
Comparison with existing literature
In the current systematic review, educational strategies were the most frequently used intervention to improve the use of antimicrobials in RACFs. Similar findings were reported by another review that evaluated quality interventions in the outpatient setting60. However, the referenced study reported some form of clinicians’ education (n=27) and patients’ education (n=18), while most studies in the current review mainly focussed on healthcare professionals’ education. The importance of multidisciplinary interventions targeting different stakeholders of the health system, including patients, has been emphasised elsewhere54. The findings of this review show that educational interventions supplemented with other strategies were more successful compared with education only interventions. Previous reviews have also highlighted the limited impact of education only strategies in ambulatory settings62, 61. This finding can be best explained by the multifactorial nature of inappropriate antibiotic prescribing that demands the implementation of multi-intervention addressing the root cause of inappropriate prescribing. Simply diverting the clinicians’ attention towards their current behaviour through audits, prescribing feedback, or suggesting an alternate behaviour may not help the cause of antimicrobial stewardship63. Similarly, the positive effects of implementing guidelines and decision support tools in RACFs can be compared with studies conducted in other settings which reported the potential of these interventions in improving antimicrobial prescribing practices66, 65, 64.
Educational interventions
Given the broad array of educational interventions, it is difficult to arrive at specifically applicable educational interventions to improve antibiotic use in RACFs. Generally, educational interventions are classified as active or passive interventions, depending on their nature and the way of implementation. Passive interventions have been least successful in improving the use of antibiotics in inpatient and ambulatory settings66, 63. However, there is a paucity of information about the supplementary effects of passive education along with other interventions. The use of reference cards to improve the knowledge of physicians and nurses was the only successful education-only intervention in this review. Though this intervention was more inclined towards a passive mode of providing education, it had the potential to shape or change behaviour of healthcare professionals towards antibiotic prescribing. The conventional way of providing the education to improve the knowledge of clinicians is not sufficient. However, if the education can effectively change the attitudes or behaviour of clinicians towards antimicrobial prescribing, it can certainly play a crucial role in antimicrobial stewardship. The similar concept of education can also be applied to education in hospital infection prevention, clinical performance improvement, and public health67. It was noted in this systematic review that education- when combined with other dynamic and interactive interventions, such as active surveillance of resistant infection, audit, and feedback- and educational training sessions resulted in significant improvement in antimicrobial use. The use of educational interventions to improve antimicrobial use in RACFs is still in its infancy stage. Overall, well-designed educational interventions have great potential to improve antimicrobial prescribing in RACFs. Future educational strategies should target the behavioral change of clinicians towards antimicrobial prescribing.
Development and implementation of local guidelines
Non-educational interventions have proven to be effective in improving antimicrobial prescribing practices. Development and implementation of guidelines were effective in decreasing antibiotic use in RACFs. However, it is important to note that two studies that supported this finding employed multifaceted interventions. Along with the implementation of guidelines, one study58 employed an interpersonal communication between a team of infectious disease experts and nursing home staff while in another study45, small group sessions were conducted with physicians and nurses about antimicrobial guidelines. Therefore, it is suggested that implementation of guidelines alone may not be effective to improve antibiotic use in a facility. Multifaceted strategies for increased dissemination, awareness, and uptake of medicines can augment the effectiveness of guidelines in strengthening antimicrobial stewardship programs.
Use of decision support tools
The use of decision support tools (RAMP and diagnostic and treatment algorithm) significantly reduced the rate of antimicrobial prescribing in RACFs. However, the use of RAMP was supplemented with a written educational material46. Besides, the use of diagnostic and treatment algorithm was employed through multifaceted interventions, including group discussions, printed educational material, outreach visits, and interviews. Caution should be made in generalizing the findings of Loeb et al. since the study only looked at the antimicrobial prescribing for UTIs. Further advancement could be made by incorporating computerized clinical decision support system in RACFs. The implementation of this system in hospital settings has shown significant improvement in reducing the use of broad-spectrum antibiotics68. The development of computer-based clinical decision support system could be beneficial for an antimicrobial stewardship program in RACFs.
Study design and quality
The use of randomized controlled trials in evaluating the effect of interventions in an antimicrobial stewardship program provides strong evidence because of the ability to account for confounding factors. The use of cluster randomized control trials also helps to improve the internal and external validity of the results; however, their design and statistical analysis are more complex than the standard patient-level randomized control trials. Most studies in this review used cluster randomized control trials but the success rate was lowest among all other study designs. The use of complex design might have played a role in the outcomes of interventions. The issue of clustering in determining the sample size was apparent in the reviewed studies. The methodological weakness including the blinding, allocation status, and adherence to guidelines on methodological quality was also observed in the reviewed studies. It is also important to discuss cross-contamination that may have occurred between intervention and control group. It can happen if the participants in both groups have close personal or professional relationship, in which intervention can be shared with the control group which may give false negative results. It is important to investigate whether or not the use of complex interventions using c-RCTs is more, less, or equally effective than standard RCTs in RACFs.
Quasi-experimental design is often used to evaluate the effects of population-level interventions, but the strength of this design varies according to design features. Therefore, it is suggested that a higher-order design should be used when conducting quasi-experiments. Interrupted time series (ITS) is considered as the most robust design for causal inference. Only one study in this review used ITS in combination with before-and-after study design. Though the implementation of successful interventions was higher in studies using QE designs, studies with stronger methodological quality are required to increase the strength of scientific evidence.
Strengths and limitations
This review provides a great insight into the published literature that evaluated the effects of interventions to improve antimicrobial use in RACFs. The review draws attention to issues which have implications for practice and research. Concurrently, the findings of this study should be viewed considering some limitations. Most studies in this review were of a moderate quality which may limit the applicability of findings in the clinical setting. Given a relatively small number of studies and a significant variation in interventions, caution should be made while interpreting the results. About half of the studies used quasi-experimental and uncontrolled before/after design that are at high risk of bias. Hence, the successful interventions using these designs should be interpreted with caution. Grey literature, unpublished studies, and articles written in languages other than English were not included in this study. Despite a comprehensive review process for study selection, the possibility to exclude relevant articles inadvertently remains.
Conclusion
This review shows that educational interventions are most effective when combined with other interactive interventions. The majority of educational interventions are aimed at improving the knowledge the health professionals about antibiotic use. Multi-faceted interventions targeting the prescribing behaviour may successfully improve antimicrobial stewardship in RACFs. Future studies should use more robust study design to enhance the quality of evidence. More research is needed to design, implement and evaluate innovative educational and non-educational stewardship interventions, and to investigate the facilitators and barriers to implementation.
Abbreviations
AMS: Antimicrobial Stewardship
AMR: Antimicrobial Resistance
RACFs: Residential Aged Care Facilities
WHO: World Health Organization
LRTIs: Lower Respiratory Tract Infections
UTIs: Urinary Tract Infections
SSTIs: Skin and Soft Tissue Infections
MeSH: Medical Subject Heading
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses
EPHPP: Effective Public Health Practice Project
ITS: Interrupted Time Series
Competing Interests
The author(s) declare that they have no competing interests.
Authors' Contributions
All the authors have participated in manuscript preparation, Manuscript review, Design, Literature search, Manuscript editing. All authors read and approved the final version of manuscript.
Acknowledgement
The authors are thankful to Institute of Postgraduate Studies (IPS) of Universiti Sains Malaysia (USM) for fellowship support.
References
-
Sharma
A.,
Antimicrobial resistance: no action today, no cure tomorrow. Indian J Med Microbiol.
2011;
29
(2)
:
91-2
.
View Article PubMed Google Scholar -
de Kraker
M.E.,
Stewardson
A.J.,
Harbarth
S.,
Will 10 million people die a year due to antimicrobial resistance by 2050?. PLoS Med.
2016;
13
(11)
:
e1002184
.
View Article PubMed Google Scholar -
Ferech
M.,
Coenen
S.,
Malhotra-Kumar
S.,
Dvorakova
K.,
Hendrickx
E.,
Suetens
C.,
Project Group
ESAC,
European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother.
2006;
58
(2)
:
401-7
.
View Article PubMed Google Scholar -
Brooks
L.,
Shaw
A.,
Sharp
D.,
Hay
A.D.,
Towards a better understanding of patients' perspectives of antibiotic resistance and MRSA: a qualitative study. Fam Pract.
2008;
25
(5)
:
341-8
.
View Article PubMed Google Scholar -
Simpson
S.A.,
Wood
F.,
Butler
C.C.,
General practitioners' perceptions of antimicrobial resistance: a qualitative study. J Antimicrob Chemother.
2007;
59
(2)
:
292-6
.
View Article PubMed Google Scholar -
Metlay
J.P.,
Strom
B.L.,
Asch
D.A.,
Prior antimicrobial drug exposure: a risk factor for trimethoprim-sulfamethoxazole-resistant urinary tract infections. J Antimicrob Chemother.
2003;
51
(4)
:
963-70
.
View Article PubMed Google Scholar -
McClean
P.,
Tunney
M.,
Gilpin
D.,
Parsons
C.,
Hughes
C.,
Antimicrobial prescribing in nursing homes in Northern Ireland: results of two point-prevalence surveys. Drugs Aging.
2011;
28
(10)
:
819-29
.
View Article PubMed Google Scholar -
McGeer
A.,
Campbell
B.,
Emori
T.G.,
Hierholzer
W.J.,
Jackson
M.M.,
Nicolle
L.E.,
Definitions of infection for surveillance in long-term care facilities. Am J Infect Control.
1991;
19
(1)
:
1-7
.
View Article PubMed Google Scholar -
Nicolle
L.E.,
Bentley
D.W.,
Garibaldi
R.,
Neuhaus
E.G.,
Smith
P.W.,
Long-Term-Care Committee
SHEA,
Antimicrobial use in long-term-care facilities. Infect Control Hosp Epidemiol.
2000;
21
(8)
:
537-45
.
View Article PubMed Google Scholar -
Peron
E.P.,
Hirsch
A.A.,
Jury
L.A.,
Jump
R.L.,
Donskey
C.J.,
Another setting for stewardship: high rate of unnecessary antimicrobial use in a veterans affairs long-term care facility. J Am Geriatr Soc.
2013;
61
(2)
:
289-90
.
View Article PubMed Google Scholar -
Cecchini
M.,
Langer
J.,
Slawomirski
L.,
Antimicrobial Resistance in G7 Countries and Beyond: Economic Issues, Policies and Options for ActionOrganization for Economic Co-operation and Development: Paris; 2015.
Google Scholar -
Organization
W.H.,
The evolving threat of antimicrobial resistance: options for actionWorld Health Organization 2012.
Google Scholar -
Conly
J.,
Antimicrobial resistance in Canada. CMAJ.
2002;
167
(8)
:
885-91
.
PubMed Google Scholar -
Davies
S.,
Gibbens
N.,
UK five year antimicrobial resistance strategy 2013 to 2018Department of Health: London; 2013.
Google Scholar -
Stuart
R.L.,
Kotsanas
D.,
Webb
B.,
Vandergraaf
S.,
Gillespie
E.E.,
Hogg
G.G.,
Prevalence of antimicrobial-resistant organisms in residential aged care facilities. Med J Aust.
2011;
195
(9)
:
530-3
.
View Article PubMed Google Scholar -
Chua
K.Y.,
Stewardson
A.J.,
Individual and community predictors of urinary ceftriaxone-resistant Escherichia coli isolates, Victoria, Australia. Antimicrob Resist Infect Control.
2019;
8
(1)
:
36
.
View Article PubMed Google Scholar -
Harris-Kojetin
L.,
Sengupta
M.,
Park-Lee
E.,
Valverde
R.,
Long-Term Care Services in the United States: 2013 Overview. Vital Health Stat 3.
2013;
:
1-107
.
-
Crnich
C.J.,
Jump
R.,
Trautner
B.,
Sloane
P.D.,
Mody
L.,
Optimizing antibiotic stewardship in nursing homes: a narrative review and recommendations for improvement. Drugs Aging.
2015;
32
(9)
:
699-716
.
View Article PubMed Google Scholar -
Montoya
A.,
Cassone
M.,
Mody
L.,
Infections in nursing homes: epidemiology and prevention programs. Clin Geriatr Med.
2016;
32
(3)
:
585-607
.
View Article PubMed Google Scholar -
Strausbaugh
L.J.,
Emerging health care-associated infections in the geriatric population. Emerg Infect Dis.
2001;
7
(2)
:
268-71
.
View Article PubMed Google Scholar -
Benoit
S.R.,
Nsa
W.,
Richards
C.L.,
Bratzler
D.W.,
Shefer
A.M.,
Steele
L.M.,
Factors associated with antimicrobial use in nursing homes: a multilevel model. J Am Geriatr Soc.
2008;
56
(11)
:
2039-44
.
View Article PubMed Google Scholar -
Warren
J.W.,
Palumbo
F.B.,
Fitterman
L.,
Speedie
S.M.,
Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc.
1991;
39
(10)
:
963-72
.
View Article PubMed Google Scholar -
Rotjanapan
P.,
Dosa
D.,
Thomas
K.S.,
Potentially inappropriate treatment of urinary tract infections in two Rhode Island nursing homes. Arch Intern Med.
2011;
171
(5)
:
438-43
.
View Article PubMed Google Scholar -
Zimmer
J.G.,
Bentley
D.W.,
Valenti
W.M.,
Watson
N.M.,
Systemic antibiotic use in nursing homes. A quality assessment. J Am Geriatr Soc.
1986;
34
(10)
:
703-10
.
View Article PubMed Google Scholar -
Pickering
T.D.,
Gurwitz
J.H.,
Zaleznik
D.,
Noonan
J.P.,
Avorn
J.,
The appropriateness of oral fluoroquinolone-prescribing in the long-term care setting. J Am Geriatr Soc.
1994;
42
(1)
:
28-32
.
View Article PubMed Google Scholar -
Loeb
M.,
Simor
A.E.,
Landry
L.,
Walter
S.,
McArthur
M.,
Duffy
J.,
Antibiotic use in Ontario facilities that provide chronic care. J Gen Intern Med.
2001;
16
(6)
:
376-83
.
View Article PubMed Google Scholar -
Vergidis
P.,
Hamer
D.H.,
Meydani
S.N.,
Dallal
G.E.,
Barlam
T.F.,
Patterns of antimicrobial use for respiratory tract infections in older residents of long-term care facilities. J Am Geriatr Soc.
2011;
59
(6)
:
1093-8
.
View Article PubMed Google Scholar -
Prasad
S.,
Smith
P.,
Meeting the threat of Antibiotic Resistance: building a new frontline defenceOffice of the Chief Scientist 2013.
Google Scholar -
Luyt
C.E.,
Bréchot
N.,
Trouillet
J.L.,
Chastre
J.,
Antibiotic stewardship in the intensive care unit. Crit Care.
2014;
18
(5)
:
480
.
View Article PubMed Google Scholar -
Velden
A. Van Der,
Duerden
M.G.,
Bell
J.,
Oxford
J.S.,
Altiner
A.,
Kozlov
R.,
Prescriber and patient responsibilities in treatment of acute respiratory tract infections essential for conservation of antibiotics. Antibiotics (Basel).
2013;
2
(2)
:
316-27
.
View Article Google Scholar -
Walker
S.,
McGeer
A.,
Simor
A.E.,
Armstrong-Evans
M.,
Loeb
M.,
Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians' and nurses' perceptions. CMAJ.
2000;
163
(3)
:
273-7
.
PubMed Google Scholar -
Schweizer
A.K.,
Hughes
C.M.,
Macauley
D.C.,
O'Neill
C.,
Managing urinary tract infections in nursing homes: a qualitative assessment. Pharm World Sci.
2005;
27
(3)
:
159-65
.
View Article PubMed Google Scholar -
Lim
C.J.,
Kwong
M.,
Stuart
R.L.,
Buising
K.L.,
Friedman
N.D.,
Bennett
N.,
Antimicrobial stewardship in residential aged care facilities: need and readiness assessment. BMC Infect Dis.
2014;
14
(1)
:
410
.
View Article PubMed Google Scholar -
Kistler
C.E.,
Sloane
P.D.,
Platts-Mills
T.F.,
Beeber
A.S.,
Khandelwal
C.,
Weber
D.J.,
Challenges of antibiotic prescribing for assisted living residents: perspectives of providers, staff, residents, and family members. J Am Geriatr Soc.
2013;
61
(4)
:
565-70
.
View Article PubMed Google Scholar -
Dartnell
J.G.,
Understanding, influencing and evaluating drug use: Therapeutic Guidelines 2001.
Google Scholar -
Duguid M, Cruickshank M. Antimicrobial stewardship in Australian hospitals 2011. Available from: https://www.safetyandquality.gov.au/wp-content/uploads/2011/01/Antimicrobial-stewardship-in-Australian-Hospitals-2011.pdf..
.
-
Rawlins
M.,
McKenzie
D.,
Mar
C.D.,
Antimicrobial stewardship: what's it all about?. Aust Prescr.
2013;
36
(4)
.
-
Smith
P.W.,
Bennett
G.,
Bradley
S.,
Drinka
P.,
Lautenbach
E.,
Marx
J.,
Society for Healthcare Epidemiology of America (SHEA)
Association for Professionals in Infection Control
Epidemiology (APIC)
SHEA/APIC Guideline: infection prevention and control in the long-term care facility. Am J Infect Control.
2008;
36
(7)
:
504-35
.
View Article PubMed Google Scholar -
Dyar
O.J.,
Pagani
L.,
Pulcini
C.,
Strategies and challenges of antimicrobial stewardship in long-term care facilities. Clin Microbiol Infect.
2015;
21
(1)
:
10-9
.
View Article PubMed Google Scholar -
Liberati
A.,
Altman
D.G.,
Tetzlaff
J.,
Mulrow
C.,
G∅tzsche
P.C.,
Ioannidis
J.P.,
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol.
2009;
62
(10)
:
e1-34
.
View Article PubMed Google Scholar -
van Gaal
B.G.,
Schoonhoven
L.,
Vloet
L.C.,
Mintjes
J.A.,
Borm
G.F.,
Koopmans
R.T.,
The effect of the SAFE or SORRY? programme on patient safety knowledge of nurses in hospitals and nursing homes: a cluster randomised trial. Int J Nurs Stud.
2010;
47
(9)
:
1117-25
.
View Article PubMed Google Scholar -
Daly
P.B.,
Smith
P.W.,
Rusnak
P.G.,
Jones
M.B.,
Giuliano
D.,
Impact on knowledge and practice of a multiregional long-term care facility infection control training program. Am J Infect Control.
1992;
20
(5)
:
225-33
.
View Article PubMed Google Scholar -
Linnebur
S.A.,
Fish
D.N.,
Ruscin
J.M.,
Radcliff
T.A.,
Oman
K.S.,
Fink
R.,
Impact of a Multidisciplinary Intervention on Antibiotic Use for Nursing Home–Acquired Pneumonia. Am J Geriatr Pharmacother.
2011;
9
(6)
:
44-50
.
View Article Google Scholar -
Schwartz
D.N.,
Abiad
H.,
DeMarais
P.L.,
Armeanu
E.,
Trick
W.E.,
Wang
Y.,
An educational intervention to improve antimicrobial use in a hospital-based long-term care facility. J Am Geriatr Soc.
2007;
55
(8)
:
1236-42
.
View Article PubMed Google Scholar -
Naughton
B.J.,
Mylotte
J.M.,
Ramadan
F.,
Karuza
J.,
Priore
R.L.,
Antibiotic use, hospital admissions, and mortality before and after implementing guidelines for nursing home-acquired pneumonia. J Am Geriatr Soc.
2001;
49
(8)
:
1020-4
.
View Article PubMed Google Scholar -
Monette
J.,
Miller
M.A.,
Monette
M.,
Laurier
C.,
Boivin
J.F.,
Sourial
N.,
Effect of an educational intervention on optimizing antibiotic prescribing in long-term care facilities. J Am Geriatr Soc.
2007;
55
(8)
:
1231-5
.
View Article PubMed Google Scholar -
Loeb
M.,
Brazil
K.,
Lohfeld
L.,
McGeer
A.,
Simor
A.,
Stevenson
K.,
Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. bmj.
2005;
331
(7518)
:
669
.
-
Baldwin
N.S.,
Gilpin
D.F.,
Tunney
M.M.,
Kearney
M.P.,
Crymble
L.,
Cardwell
C.,
Cluster randomised controlled trial of an infection control education and training intervention programme focusing on meticillin-resistant Staphylococcus aureus in nursing homes for older people. J Hosp Infect.
2010;
76
(1)
:
36-41
.
View Article PubMed Google Scholar -
Fleet
E.,
Gopal Rao
G.,
Patel
B.,
Cookson
B.,
Charlett
A.,
Bowman
C.,
Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother.
2014;
69
(8)
:
2265-73
.
View Article PubMed Google Scholar -
Zimmerman
S.,
Sloane
P.D.,
Bertrand
R.,
Olsho
L.E.,
Beeber
A.,
Kistler
C.,
Successfully reducing antibiotic prescribing in nursing homes. J Am Geriatr Soc.
2014;
62
(5)
:
907-12
.
View Article PubMed Google Scholar -
Pettersson
E.,
Vernby
A.,
Mölstad
S.,
Lundborg
C.S.,
Can a multifaceted educational intervention targeting both nurses and physicians change the prescribing of antibiotics to nursing home residents? A cluster randomized controlled trial. J Antimicrob Chemother.
2011;
66
(11)
:
2659-66
.
View Article PubMed Google Scholar -
Hasson
H.,
Arnetz
J.E.,
The impact of an educational intervention for elderly care nurses on care recipients' and family relatives' ratings of quality of care: a prospective, controlled intervention study. Int J Nurs Stud.
2008;
45
(2)
:
166-79
.
View Article PubMed Google Scholar -
van Buul
L.W.,
van der Steen
J.T.,
Achterberg
W.P.,
Schellevis
F.G.,
Essink
R.T.,
de Greeff
S.C.,
Effect of tailored antibiotic stewardship programmes on the appropriateness of antibiotic prescribing in nursing homes. J Antimicrob Chemother.
2015;
70
(7)
:
2153-62
.
View Article PubMed Google Scholar -
Rummukainen
M.L.,
Jakobsson
A.,
Matsinen
M.,
Järvenpää
S.,
Nissinen
A.,
Karppi
P.,
Reduction in inappropriate prevention of urinary tract infections in long-term care facilities. Am J Infect Control.
2012;
40
(8)
:
711-4
.
View Article PubMed Google Scholar -
Mody
L.,
Krein
S.L.,
Saint
S.,
Min
L.C.,
Montoya
A.,
Lansing
B.,
A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med.
2015;
175
(5)
:
714-23
.
View Article PubMed Google Scholar -
Zabarsky
T.F.,
Sethi
A.K.,
Donskey
C.J.,
Sustained reduction in inappropriate treatment of asymptomatic bacteriuria in a long-term care facility through an educational intervention. Am J Infect Control.
2008;
36
(7)
:
476-80
.
View Article PubMed Google Scholar -
Jump
R.L.,
Olds
D.M.,
Seifi
N.,
Kypriotakis
G.,
Jury
L.A.,
Peron
E.P.,
Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol.
2012;
33
(12)
:
1185-92
.
View Article PubMed Google Scholar -
Thomas
B.,
Ciliska
D.,
Dobbins
M.,
Micucci
S.,
Quality Assessment Tool for Quantitative Studies Dictionary: The Effective Public Health Practice Project (EPHPP)McMaster University 2008.
Google Scholar -
Schwartz
D.N.,
Abiad
H.,
DeMarais
P.L.,
Armeanu
E.,
Trick
W.E.,
Wang
Y.,
An Educational Intervention to Improve Antimicrobial Use in a Hospital-Based Long-Term Care Facility: (See Editorial Comments by Dr. Lona Mody on pp 1301\textendash1302). J Am Geriatr Soc.
2007;
55
(8)
:
1236-42
.
View Article PubMed Google Scholar -
Ranji
S.R.,
Steinman
M.A.,
Shojania
K.G.,
Sundaram
V.,
Lewis
R.,
Arnold
S.,
Closing the quality gap: a critical analysis of quality improvement strategies. vol. 4: antibiotic prescribing behaviorTechnical Reviews, No. 9.4 2006.
Google Scholar -
Giguère
A.,
Légaré
F.,
Grimshaw
J.,
Turcotte
S.,
Fiander
M.,
Grudniewicz
A.,
Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews.
2012;
2010
(10)
.
View Article Google Scholar -
Ivers
N.,
Jamtvedt
G.,
Flottorp
S.,
Young
J.M.,
Odgaard-Jensen
J.,
French
S.D.,
Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev.
2012;
6
(6)
:
000259
.
View Article PubMed Google Scholar -
Arnold
S.R.,
Straus
S.E.,
Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane database of systematic reviews.
2005;
2005
(4)
.
View Article Google Scholar -
Samore
M.H.,
Bateman
K.,
Alder
S.C.,
Hannah
E.,
Donnelly
S.,
Stoddard
G.J.,
Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. JAMA.
2005;
294
(18)
:
2305-14
.
View Article PubMed Google Scholar -
Sintchenko
V.,
Iredell
J.R.,
Gilbert
G.L.,
Coiera
E.,
Handheld computer-based decision support reduces patient length of stay and antibiotic prescribing in critical care. J Am Med Inform Assoc.
2005;
12
(4)
:
398-402
.
View Article PubMed Google Scholar -
Davey
P.,
Brown
E.,
Charani
E.,
Fenelon
L.,
Gould
I.M.,
Holmes
A.,
Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev.
2013;
4
(4)
:
003543
.
View Article PubMed Google Scholar -
Tamma
P.,
Antimicrobial Stewardship, An Issue of Infectious Disease ClinicsElsevier Health Sciences 2014.
Google Scholar -
Yong
M.K.,
Buising
K.L.,
Cheng
A.C.,
Thursky
K.A.,
Improved susceptibility of Gram-negative bacteria in an intensive care unit following implementation of a computerized antibiotic decision support system. J Antimicrob Chemother.
2010;
65
(5)
:
1062-9
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 1 (2020)
Page No.: 3550-3562
Published on: 2020-01-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5929 times
- Download PDF downloaded - 1145 times
- Appendix downloaded - 954 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



