Abstract
Introduction: Imbalance between total energy intake and expenditure causes accumulation of excess fat and sugar in the body which leads to development of diabetes mellitus type II, obesity, and metabolic syndrome. These harmful diseases accelerate aging and cause fatal metabolic disorders as people age. Inhibition of pancreatic lipase, and alpha glucosidase digestive enzymes is a step that can reduce excess fat and sugar from the body, which is an essential component of healthy aging.
Methodology: In this study, aqueous and 50% ethanolic extracts of Hibiscus sabdariffa were investigated for their inhibitory activities on pancreatic lipase and alpha-glucosidase, in addition to their antioxidant activities (using UV-vis spectrophotometer).
Results: Both extracts displayed antioxidant properties, indicated by IC50 of 5166.80 mg/mL for H. sabdariffa aqueous extract and 2809.10 mg/mL for H. sabdariffa 50% ethanolic extract. The extracts also suppressed the activities of pancreatic lipase and alpha-glucosidase enzymes, which suggests possible antiobesity and anti-diabetic activities. H. sabdariffa aqueous extract inhibited pancreatic lipase activity with IC50 of 167.5+/-12.7 mg/mL, whereas H. sabdariffa 50% ethanolic extract inhibited the enzyme with an IC50 of 790.65+/-16.02 mg/mL. Both H. sabdariffa aqueous and ethanolic extracts also successfully inhibited alpha-glucosidase enzyme activity with IC50 949.88 +/-10.83 mg/mL, and 378.33 +/-4.20 mg/mL, respectively.
Conclusion: Taken together, the outcome of the investigations offers the possibility of the extracts as an anti-obesity, anti-diabetic, anti-metabolic and anti-aging agent, which can be developed into supplements for adults to prevent the occurrence of these prevalent diseases and delay the onset and effects of aging.
Introduction
Malaysia will be an aging country by 2020 where 7% of the country’s population will be at the age of 65 or above1, and in just 23 years after that, the Malaysian aged population will double to 14%2. Ensuring physical health and well-being of older adults is crucial, in part, to reduce incidences of ill health and hospitalization so that people can actively participate in society. Biologically, aging is a time-related functional decline of various organs and tissues that are involved in survival and fertility of an organism. It is mainly caused by oxidative damage3 and metabolic dysregulation4.
Older adults are burdened with the development of fatal metabolic related diseases, such as cardiovascular disorders, high blood pressure, type II diabetes mellitus, insulin resistance, hyperlipidemia, hypercholesterolemia, and obesity. Metabolic syndrome is defined as a cluster of diseases which are associated with the development of cardiovascular disorders5, mainly caused by accumulation of excess fat and sugar in the body. The pervasiveness of metabolic syndromes rises with age7, 6. Development of metabolic syndrome is 15% higher in patients above the age of 40 compared to the younger ones8. For every year of increase in age, the risk of metabolic syndrome increases by 3%9. Obesity is the most significant marker for development of metabolic syndrome10. Obesity also accelerates aging, and incremental increases in the adipocyte level increases systemic ROS levels4. In short, accumulation of excess fat and sugar is one of the factors leading to aging symptoms and most metabolic-related problems that arise as one ages.
Natural products are well-known for their synergistic action of bioactive compounds, thereby facilitating a more anti-oxidative and multi-targeted approach compared to currently available single drugs11. Hibiscus sabdariffa is a medicinal plant well-known for its therapeutic values, including anti-hyperglycemic, anti-hypercholesteremic, and thermogenesis-enhancement potential of its aqueous extract12. It is also rich in bioactive compounds, including delphinidin-3-glucoside, cyanidin-3-glucoside, delphinidin-3-sambubioside, and cyanidin-3-sambubioside13. Delphinidins possess numerous pharmacological activities, such as antioxidant, anti-inflammatory, anti-cancer, and anti-obesity properties14. Delphinidins inhibit adipogenesis and anti-obesity attributes16, 15. Anthocyanidins have anti-cancer, anti-diabetic and anti-obesity properties17, and cyanidin-3-glucoside has also been reported to have good antioxidative capacity18.
Targeting a plant which has synergistic antioxidant, alpha-glucosidase enzyme and pancreatic lipase enzyme inhibitory effects can delay the onset of biological aging and minimize glucose and fat absorption for preventing development of metabolic-related diseases. Thus, plant-based therapy may be a reliable approach to improve the general health and well-being of older adults. Hence, the current research study aims to evaluate the total phenolic and flavonoid contents of H. sabdariffa calyx extracts, as well as its antioxidant, alpha-glucosidase and pancreatic lipase inhibitory properties, to determine the potential of H. sabdariffa as an anti-aging health supplement.
Materials and Methods
Extraction
One liter of extracting solvent (distilled water for aqueous extract and 50% ethanol for ethanolic extract) was added to 100 g H. sabdariffa calyx (purchased from Herbagus Sdn. Bhd., Pulau Pinang; collected from Kepala Batas, Pulau Pinang area in October 2016.), sonicated for 1 hour, and macerated under dark condition for 24 h at 20°C ± 5. The mixture was then filtered with muslin cloth to separate plant material from fluid extract. The fluid extract was re-filtered using a rotary vacuum filter to remove any remaining coarse material. For aqueous extract, the filtered fluid extract was spray dried using Mini Spray Dryer B-290 (BUCHI, New Castle) at inlet temperature of 80°C, aspirator rate of 100% and flow rate of 5%. For 50% ethanolic extract, the filtered fluid extract was diluted with distilled water to 20:80 ethanol to water ratio prior to spray drying.
Determination of total phenolics
Total phenolic content (TPC) of H. sabdariffa aqueous and 50% ethanolic extract was determined using Folin-Ciocalteu (FC) reagent (Sigma Aldrich, St. Louis, MO) and gallic acid (Sigma Aldrich, St. Louis, MO), per standard protocol with slight modifications. In a test tube, 400 µL of 1 mg/mL H. sabdariffa extract was added to 2 mL FC reagent. After incubating in the dark at 20°C±5 for 5 minutes, 1.6 mL of 7.5% sodium carbonate (Sigma Aldrich) was added into the mixture. The final mixture was incubated in the dark at 20°C±5. The absorbance was measured at 765 nm using an UV-Vis spectrophotometer. A calibration curve of gallic acid standard was constructed. All results are expressed as mg gallic acid extract (GAE) per g extract.
Determination of total flavonoids
Total flavonoid contents were determined by the aluminum calorimetric method19. In a 96-well plate, 150 µL of 1 mg/mL H. sabdariffa extract was added to 150 µL (2%) aluminum chloride (AlCl3) (Sigma-Aldrich). After incubating for 15 min at 20°C±5, the absorbance was measured at 435 nm using a spectrophotometer. A calibration curve of quercetin (Sigma-Aldrich) standard was constructed. All results were expressed as mg quercetin extract (QE) per g extract.
Antioxidant activity determination
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich) free radical scavenging capacity of H. sabdariffa aqueous and 50% ethanolic extracts was evaluated according to Chin (2012)20. H. sabdariffa aqueous and 50% ethanolic extracts were serially diluted at a dilution factor of 2- from 2000 µg/mL to 125 µg/mL. Ascorbic acid was also serially diluted from 125 µg/mL to 1.95 µg/mL, at a dilution factor of 2. Fifty µL of extract (or standard) was then added to 950 µL of 200 µM ethanolic DPPH solution, vortexed, and incubated at 37°C for 30 min. The absorbance of the mixtures at various concentrations was measured at 517 nm using an UV-Vis spectrophotometer. The IC50 was analyzed using OriginPro software version 8.1 (Northampton, Massachusetts).
In vitro pancreatic lipase inhibitory assay
Firstly, 50 µL of extract or orlistat standard (Cayman Chemical Company, Michigan, USA) was added to 100 µL of 0.1 mg/mL porcine pancreatic lipase (Sigma Aldrich) in pH 6.8 potassium phosphate buffer solution. The mixture was incubated at 37°C for 30 min. Then, 50µL of 2.275 mM p-NPB (Sigma-Aldrich) in dimethyl formamide was added to the mixture and incubated at 37°C for 30 min. The final concentration of extracts in the reaction media was 2500 µg/mL, 1250 µg/mL, 625 µg/mL, 312.5 µg/mL, 156.25 µg/mL, 78 µg/mL, and 39.06 µg/mL. The enzyme inhibitory activity of the plant extracts was calculated using the following equation, where A is the absorbance. The IC50 was analyzed using OriginPro software version 8.1 (Northampton, Massachusetts)21.
In vitro alpha-glucosidase enzyme inhibitory assay
The potency of the plant extracts in alpha-glucosidase enzyme inhibitory activity was evaluated by measuring the hydrolysis of p-NPG to p-nitrophenol ion and glucose with minor modifications. Briefly, 5000 to 78.13 µg/mL acarbose standard solution was prepared by serial dilution with dilution factor of 2. Then, 10 mg/mL of extract was prepared and serially diluted from 10,000 µg/mL to 156 µg/mL, at a dilution factor of 2, to prepare the working solutions. In a 96-well microplate, 50 µL of sample or acarbose positive control (Bayer, Petaling Jaya, Malaysia) was added to 100 µL of 1 U/mL alpha-glucosidase from Saccharomyces cerevisiae (Sigma-Aldrich) solution in potassium phosphate buffer pH 6.8, and incubated for 10 min at 25ºC. Then, 50 µL of 5 mM p-NPG (Sigma-Aldrich) was added to each well and incubated at 25ºC for 5 min before reading the absorbance at 405 nm on an UV-vis spectrophotometer. The final concentration of extracts in the reaction media was 2500 µg/mL, 1250 µg/mL, 625 µg/mL, 312.5 µg/mL, 156.25 µg/mL, 78 µg/mL, and 39.06 µg/mL. The enzyme inhibitory activity of the plant extracts was calculated using the following equation, where A is the absorbance. IC50 was analyzed using OriginPro software (OriginLab, Northampton, MA, USA)22.
Statistical analyses
All analyses were performed in triplicate and data was reported as mean ± SEM. One-way ANOVA was performed to assess the significant differences at p<0.05. All analyses were carried out using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA). The IC50 was analyzed using Origin Pro software.
Results
Determination of total phenolics and total flavonoids
The total phenolic and flavonoid content in the extracts were determined by comparing them with gallic acid and quercetin standards, respectively. The total phenolics and flavonoids in the two extracts are reported in Table 1 as mg gallic acid/g extract and mg Quercetin/g extract, respectively. The phenolic and flavonoid content in H. sabdariffa 50% ethanolic extract was significantly higher than in H. sabdariffa aqueous extract. Indeed, H. sabdariffa 50% ethanolic extract contained total phenolics of 15.56 ± 0.02 mg GAE/g and total flavonoids of 3.53 ± 0.00 mg QE/g, whereas H. sabdariffa aqueous extract contained total phenolics and flavonoids of 6.65 ± 0.00 mg GAE/g and 10.08±0.01 mg QE/g, respectively.
| Extracts | Total phenolics (mg) | Total flavonoids (mg) |
|---|---|---|
| H. sabdariffa aqueous extrac | 6.65±0.000 | 3.53±0.002 |
| H. sabdariffa 50% ethanolic extract | 15.56±0.023 | 10.08±0.012 |
Antioxidant activity determination
The IC50 of H. sabdariffa aqueous and ethanolic extracts were determined through DPPH method, as reported in Table 2. H. sabdariffa 50% ethanolic extract exhibited lower IC50 (280±9.1 µg/mL), compared to aqueous extract which exhibited an IC50 of 516±6.8 µg/mL.
| Extract | IC50±SEM (µg/mL) |
|---|---|
| Ascorbic acid | 42.74±2.6 |
| H. sabdariffa aqueous extract | 516±6.80 |
| H. sabdariffa 50% ethanolic extract | 280±9.10 |
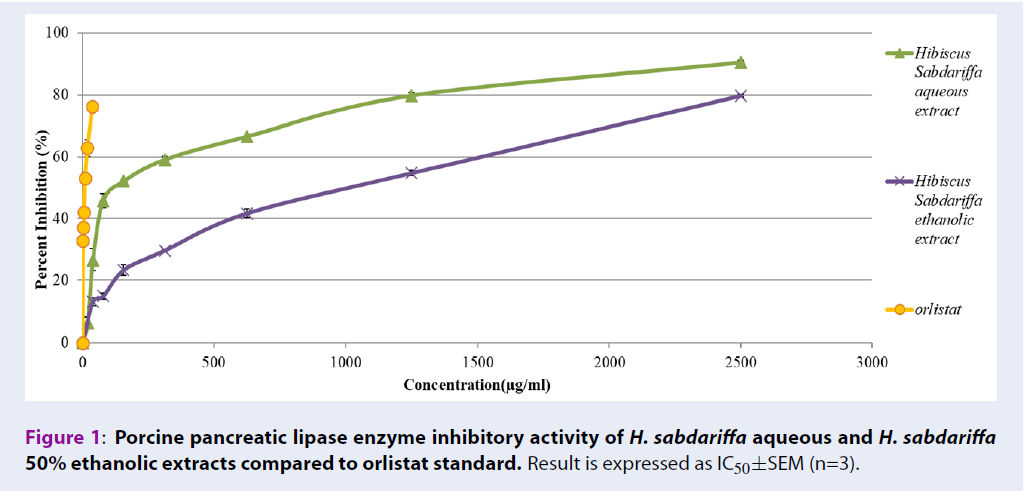
In vitro pancreatic lipase enzyme inhibitory assay
Table 3 shows the pancreatic lipase enzyme inhibitory activities of H. sabdariffa extracts. The assay was performed using orlistat as a positive control which showed an IC50 of 6.39±0.14 µg/mL. H. sabdariffa aqueous extract inhibited pancreatic lipase enzyme from 6.36±0.75% to 90.48±0.34% (with IC50 167.51±2.7 µg/mL), compared to H. sabdariffa 50% ethanolic extract which inhibited from 12.95±1.34 µg/mL to 79.76±0.49 µg/mL (with IC50 790.65±16.02 µg/mL).
| Extract | IC50±SEM (µg/mL) |
|---|---|
| Orlistat | 6.39±0.14 |
| H. sabdariffa aqueous extract | 167.51±2.7 |
| H. sabdariffa 50% ethanolic extract | 790.65±16.02 |
In vitro alpha glucosidase enzyme inhibitory assay
The inhibitory activities of H. sabdariffa extracts against alpha-glucosidase enzyme is reported in Table 4. Acarbose was used as a positive control and showed IC50 of 75.71±0.96 µg/mL. H. sabdariffa aqueous extract inhibited alpha glucosidase enzyme from 2.43±0.4% to 74.99±0.29% (with IC50 949.88±10.83), compared to H. sabdariffa 50% ethanolic extract which inhibited from 5.34±0.33 to 86.11±0.15 (with IC50 378.33±4.2 µg/mL).
| Extract | IC50±SEM (µg/mL) |
|---|---|
| Acarbose | 75.71 ± 0.96 |
| H. sabdariffa aqueous extract | 949.88 ± 10.83 |
| H. sabdariffa 50% ethanolic extract | 378.33 ± 4.20 |
Discussion
Imbalance between total energy intake and expenditure causes accumulation of excess fat and sugar in the body which leads to obesity, type II diabetes mellitus, metabolic syndrome, and accelerated aging. The prevalence of obesity, diabetes mellitus type II, and metabolic syndromes also increases as one ages. Hence, inhibiting the activity of alpha glucosidase and pancreatic lipase enzyme activity may be a promising approach in overcoming problems related to accumulation of excess fat and sugar as one ages. Simultaneously, high content of antioxidants also slows down the aging process. Thus, the current study investigated the antioxidant, anti-pancreatic lipase and anti-alpha glucosidase activities of Hibiscus sabdariffa aqueous and 50% ethanolic extract.
The extract is targeted to be delivered in the intestines to inhibit the dietary enzymes and to be absorbed into the circulatory system to act as antioxidants. Thus, it is most suitable to be delivered as an oral dose form. Water and ethanol are two solvents which are least toxic compared to others for the development of oral dose form. Extraction with water and 50% ethanol showed high yield compared to 100% ethanolic extract. Thus, H. sabdariffa calyx was extracted with water and 50% ethanol, respectively, to study their antioxidant, anti-pancreatic lipase, and anti-alpha glucosidase potency.
ROS are generated from biochemical reactions and dietary xenobiotics. They initiate chain reaction and cause oxidative stress which cause damage to cells and organs. Antioxidants inhibit oxidation, terminate chain reaction, prevent cell damage, thereby delaying aging. Plants are rich with bioactive compounds, mainly phenolics, which are well-known for antioxidant properties. The antioxidant activities of H. sabdariffa extracts was determined using TPC, TFC, and DPPH assays. Both extracts were found to contain significant amount of phenolics and flavonoids, and to exhibit antioxidant activity against DPPH. However, H. sabdariffa 50% ethanolic extract contain higher phenolics and flavonoids compared to the aqueous extract. Corresponding to the phenolic and flavonoid contents, the antioxidant activity was also observed to be higher in 50% ethanolic extract (280±9.10 µg/mL).
Accumulation of fat increases adipose tissue mass and ROS levels, which initiate a chain reaction leading to damage of organs and acceleration of aging. Moreover, the prevalence of metabolic disorders due to accumulation of excess fat and sugar among older people are high. Thus, inhibiting the activity of alpha glucosidase and pancreatic lipase dietary enzymes may reduce absorption of fat and glucose in the body, and prevent accumulation of fat and generation of ROS. In addition, metabolic disorders due to accumulation of excess fat and sugar among older people can be solved. Alpha-glucosidase enzyme breaks down large polysaccharides into simpler monosaccharides or disaccharides. Similarly, pancreatic lipases break down large triglycerides into simpler glycerol and fatty acid. High activities of these enzymes enhance the absorption of sugar and fat into the systemic circulatory system. Therefore, inhibiting the activities of these enzymes is a key target to minimize fat and sugar absorption, and is a widely studied mechanism to investigate the potency of natural products, such as anti-obesity agents23. Orlistat, a potent pancreatic lipase inhibitor has been shown to prevent 30% of dietary fat absorption from the intestines into the bloodstream24. However, it is also associated with side effects including abdominal cramping, fat-soluble vitamin deficiencies, liquid stools, and fecal urgency25. Current research indicates that both H. sabdariffa aqueous and ethanolic extracts have high antioxidant activity and successfully inhibit pancreatic lipase and alpha-glucosidase enzyme activities. However, H. sabdariffa aqueous extract showed higher potency in inhibiting pancreatic lipase enzyme with an IC50 of 167.51±2.7 µg/mL (Figure 1). It inhibits the enzyme from 6.36% to 90.48%. On the other hand, H. sabdariffa 50% ethanolic extract inhibited alpha-glucosidase with IC50 of 378.33±4.20 µg/mL (Figure 2). Thus, both extracts show good candidacy to be developed into antioxidant supplements to eliminate excess fat and sugar from the body and to slow down the process of aging.
The phytochemicals in plant extracts, including phenolic compounds, flavonoids, anthocyanidins and other compounds, have potent alpha glucosidase and pancreatic lipase enzyme inhibitory potential26 . For instance, ethyl acetate extract of Vitis rotundifolia exhibited potent alpha-glucosidase and pancreatic lipase inhibitory activity27. Similarly, alpha-glucosidase and pancreatic lipase enzyme inhibitory activity of Nelumbo nucifera leaves was also reported28. Natural antioxidants including polyphenols, ascorbic acid and tocopherols have also been reported to have pancreatic lipase inhibitory effect29. Some flavanols have also been reported to be potent inhibitors of pancreatic lipase activity31, 30. Belfeki et al. (2016) also reported a strong positive correlation between antioxidant capacity and digestive enzyme inhibitory activities of several plants32.
Conclusion
Most complications, including metabolic syndromes that develop as one ages, are caused by accumulation of excess sugar and fat in the body. Accumulation of fat in adipocytes elevates oxidative damage and accelerates aging. Current research revealed that H. sabdariffa aqueous and ethanolic extracts contain high phenolic content and have good therapeutic values, including antioxidative, anti-pancreatic lipase and anti-alpha-glucosidase activities. Hence, it can be a good alternative for elimination of excess fat and sugar from the body and can be a potential candidate to develop as supplements or functional food that could contribute to anti-aging, anti-obesity, anti-diabetic and anti-metabolic syndrome for older adults. However, this is a preliminary in vitro study and requires further investigations, including in vivo assays and human trials.
Abbreviations
DMF: Dimethyl formamide
DPPH: 2,2-diphenyl-1-picrylhydrazyl
FC: Follin- Ciocalteau’s
GAE: Gallic acid equivalent
IC50: 50% inhibitory concentration
p-NPB: p-Nitrophenyl butyrate
p-NPG: p-Nitrophenyl glucopyranoside
QE: Quercetin equivalent
ROS: Reactive oxygen species
TFC: Total flavonoid content
TPC: Total phenolic content
Competing Interests
The authors declare no conflict of interest.
Authors' Contributions
Zuliana Ridzwan designed the experiment, conducted primary literature search, carried out pancreatic lipase and alpha-glucosidase inhibitory assay and drafted the manuscript. Nurzalina Abdul Karim Khan was the project leader who supervised the research and managed the grant sponsorship. Insathe Mohd Ali carried out total phenolics and total flavonoids determining assays. Mohd Dayoob carried out the extraction of Hibiscus sabdariffa calyx. Shahad Hussein conducted antioxidant assay.
Acknowledgments
This research was supported by a grant from the National Institutes of Biotechnology Malaysia, PFARMASI/304/K105/650904.
References
-
Sen Tyng
C.,
Aizan Hamid
T.,
Population ageing and the Malaysian chinese: issues and challenges. Malaysian J Chinese Stud..
2015;
4
(1)
:
1-13
.
-
Baharin
R.,
Saad
S.,
Ageing population and health care expenditure: evidence using time series analysis. Malaysian J Soc Sp..
2018;
14
(4)
:
65-73
.
View Article Google Scholar -
Finkel
T.,
Holbrook
N.J.,
Oxidative Stress, aging. Nature.
2000;
408
(Nov)
:
239-47
.
View Article PubMed Google Scholar -
Ahima
R.S.,
Connecting obesity, aging and diabetes. Nat Med.
2009;
15
(9)
:
996-7
.
View Article PubMed Google Scholar -
Eckel
R.H.,
Grundy
S.M.,
Zimmet
P.Z.,
The metabolic syndrome. Lancet.
2005;
365
(9468)
:
1415-28
.
View Article PubMed Google Scholar -
Mohamud
W.N.,
Ismail
A.,
Khir
A.S.,
Ismail
I.S.,
Musa
K.I.,
Kadir
K.A.,
Prevalence of metabolic syndrome and its risk factors in adult Malaysians: results of a nationwide survey. Diabetes Res Clin Pract.
2012;
96
(1)
:
91-7
.
View Article PubMed Google Scholar -
Tan
W.S.,
Ng
C.J.,
Khoo
E.M.,
Low
W.Y.,
Tan
H.M.,
The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male.
2011;
14
(4)
:
231-6
.
View Article PubMed Google Scholar -
Lim
K.G.,
Cheah
W.K.,
A review of metabolic syndrome research in Malaysia. Med J Malaysia.
2016;
71
(June)
:
20-8
.
PubMed Google Scholar -
Chee
H.P.,
Hazizi
A.S.,
Barakatun Nisak
M.Y.,
Mohd Nasir
M.T.,
Metabolic risk factors among government employees in Putrajaya, Malaysia. Sains Malays.
2014;
43
(8)
:
1165-74
.
-
Després
J.P.,
Lemieux
I.,
Abdominal obesity and metabolic syndrome. Nature.
2006;
444
(7121)
:
881-7
.
View Article PubMed Google Scholar -
Brusotti
G.,
Cesari
I.,
Dentamaro
A.,
Caccialanza
G.,
Massolini
G.,
Isolation and characterization of bioactive compounds from plant resources: the role of analysis in the ethnopharmacological approach. J Pharm Biomed Anal.
2014;
87
:
218-28
.
View Article PubMed Google Scholar -
Mohamed
G.A.,
Ibrahim
S.R.,
Elkhayat
E.S.,
El
D.R.,
Natural anti-obesity agents. Bull Fac Pharm Cairo Univ.
2014;
52
(2)
:
269-84
.
View Article Google Scholar -
Jabeur
I.,
Pereira
E.,
Barros
L.,
Calhelha
R.C.,
Soković
M.,
Oliveira
M.B.,
Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res Int.
2017;
100
(Pt 1)
:
717-23
.
View Article PubMed Google Scholar -
Patel
K.,
Jain
A.,
Patel
D.K.,
Medicinal significance, pharmacological activities, and analytical aspects of anthocyanidins `delphinidin': A concise report. J Acute Dis.
2013;
2
(3)
:
169-78
.
View Article Google Scholar -
Harada
G.,
Onoue
S.,
Inoue
C.,
Hanada
S.,
Katakura
Y.,
Delphinidin-3-glucoside suppresses lipid accumulation in HepG2 cells. Cytotechnology.
2018;
70
(6)
:
1707-12
.
View Article PubMed Google Scholar -
Rahman
N.,
Jeon
M.,
Kim
Y.S.,
Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/β-catenin signaling. Biofactors.
2016;
42
(1)
:
49-59
.
PubMed Google Scholar -
Lee
Y.M.,
Yoon
Y.,
Yoon
H.,
Park
H.M.,
Song
S.,
Yeum
K.J.,
Dietary anthocyanins against obesity and inflammation. Nutrients.
2017;
9
(10)
:
1-15
.
View Article PubMed Google Scholar -
Tsuda
T.,
Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants.
2016;
5
(2)
:
13
.
-
Quettier-Deleu
C.,
Gressier
B.,
Vasseur
J.,
Dine
T.,
Brunet
C.,
Luyckx
M.,
Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol.
2000;
72
(1-2)
:
35-42
.
View Article PubMed Google Scholar -
Chin LS. Investigation of skin anti-ageing effects of three Chromolaena odorata ethanol extracts. Universiti Sains malaysia; 2012. .
.
-
Supkamonseni
N.,
Thinkratok
A.,
Meksuriyen
D.,
Srisawat
R.,
Hypolipidemic and hypoglycemic effects of Centella asiatica ( L .) extract in vitro and in vivo. 2014;
52
(Oct)
:
965-971
.
-
Johnson
M.H.,
de Mejia
E.G.,
Fan
J.,
Lila
M.A.,
Yousef
G.G.,
Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol Nutr Food Res.
2013;
57
(7)
:
1182-97
.
View Article PubMed Google Scholar -
Marrelli
M.,
Loizzo
M.R.,
Nicoletti
M.,
Menichini
F.,
Conforti
F.,
In vitro investigation of the potential health benefits of wild Mediterranean dietary plants as anti-obesity agents with α-amylase and pancreatic lipase inhibitory activities. J Sci Food Agric.
2014;
94
(11)
:
2217-24
.
View Article PubMed Google Scholar -
Ado
M.A.,
Abas
F.,
Mohammed
A.S.,
Ghazali
H.M.,
Anti- and pro-lipase activity of selected medicinal, herbal and aquatic plants, and structure elucidation of an anti-lipase compound. Molecules.
2013;
18
(12)
:
14651-69
.
View Article PubMed Google Scholar -
Lunagariya
N.A.,
Patel
N.K.,
Jagtap
S.C.,
Bhutani
K.K.,
Inhibitors of pancreatic lipase: state of the art and clinical perspectives. EXCLI J.
2014;
(13)
:
897-921
.
-
Rahim
A.T.,
Takahashi
Y.,
Yamaki
K.,
Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res Int.
2015;
75
:
289-94
.
View Article PubMed Google Scholar -
You
Q.,
Chen
F.,
Wang
X.,
Jiang
Y.,
Lin
S.,
Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. Lebensm Wiss Technol.
2012;
46
(1)
:
164-8
.
View Article Google Scholar -
Liu
S.,
Li
D.,
Huang
B.,
Chen
Y.,
Lu
X.,
Wang
Y.,
Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J Ethnopharmacol.
2013;
149
(1)
:
263-9
.
View Article PubMed Google Scholar -
Gooda Sahib
N.,
Abdul Hamid
A.,
Saari
N.,
Abas
F.,
Pak Dek
M.S.,
Rahim
M.,
Anti-pancreatic lipase and antioxidant activity of selected tropical herbs. Int J Food Prop.
2012;
15
(3)
:
569-78
.
View Article Google Scholar -
Dechakhamphu
A.,
Wongchum
N.,
Screening for anti-pancreatic lipase properties of 28 traditional Thai medicinal herbs. Asian Pac J Trop Biomed.
2015;
5
(12)
:
1042-5
.
View Article Google Scholar -
Sergent
T.,
Vanderstraeten
J.,
Winand
J.,
Beguin
P.,
Schneider
Y.J.,
Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem.
2012;
135
(1)
:
68-73
.
View Article Google Scholar -
Belfeki
H.,
Mejri
M.,
Hassouna
M.,
Antioxidant and anti-lipases activities in vitro of Mentha viridis and Eucalyptus globulus extracts. Ind Crops Prod.
2016;
89
:
514-21
.
View Article Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 1 (2020)
Page No.: 3572-3578
Published on: 2020-01-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5829 times
- Download PDF downloaded - 1367 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress



