Abstract
Background: Pulmonary regurgitation (PR) is often known as an acquired condition after surgical correction of tetralogy of Fallot (TOF). Therefore, the present study aimed to compare the use of monocusp valve (MV) implantation and transannular patch (TAP) angioplasty on PR and right ventricular (RV) failure following surgery to repair TOF.
Methods: This prospective randomized clinical trial (RCT) was performed on a total number of 60 patients undergoing reconstructive surgery on TOF. For this purpose, TAPs without and with monocusp reconstruction were used in Group I (n = 30 patients) and Group II (n = 30 patients), respectively. Then, echocardiographic parameters, mortality rates, and clinical data from pediatric intensive care unit (PICU) were evaluated during a follow-up period for both groups.
Results: Out of the 60 patients undergoing surgery and evaluated, 39 individuals were male (65%) and the rest were female (n = 21 patients, 35%). No significant difference was observed in terms of age, body weight, body surface area (BSA), mortality rate, and ejection fraction (EF) between the two study groups. The findings revealed that the number of patients with severe PR was higher in the group receiving TAP angioplasty. Furthermore, the difference between the two groups with regards to severity of PR was significant (p = 0.012).
Conclusion: It was concluded that MV reconstruction of TOF is effective in reducing pulmonary artery (PA) and pulmonary valve (PV) insufficiency.
Background
Tetralogy of Fallot (TOF) is known as a congenital heart disease (CHD) impairing oxygen delivery to the tissues. It includes four types of defects that often occur together, i.e., overriding aorta, pulmonary stenosis, ventricular septal defect (VSD), and right ventricular hypertrophy (RVH)1, 2. Former surgical procedures to correct this congenital anomaly have been thus far accompanied by a large right ventriculotomy to close ventricular septal defect (VSD) and pulmonary stenosis, resolved through transannular patch enlargement (TAPE)3, 4. However, today, ventriculotomy is less commonly exercised to close VSD; other methods such as transarterial VSD closure and transarterial right ventricular outflow tract (RVOT) resection or patch plasty, together with transarterial transpulmonary repair, have been developed5. One of the important advantages of TAP is that it immediately leads to RV hypertension reduction as RVOT can usually grow proportionally with a child6, 7. Nevertheless, TOF reconstruction with a TAP can induce post-surgery pulmonary regurgitation (PR). Therefore, reduction in cardiac output due to early RV dysfunction poses a myriad of problems for these patients. However, this is not the only cause of early morbidity and mortality after surgery. Indeed, other factors such as age and weight at the time of surgery, surgeons’ skills, use of TAP, duration of surgery, and cardiopulmonary bypass (CPB) time may also have impact8, 9, 10. Despite advances and further developments of novel techniques in this field, long-term mortality and PR are still at high rates in patients undergoing TAP repair11. To diminish volume overload, even for a short time after surgery, some studies have recommended implanting a monocusp valve (MV)12, 13, 14. Theoretically, a monocusp patch can mitigate patch-related PR although long-term results might not be satisfactory, especially in younger children15. Accordingly, this work aimed to evaluate the results of two surgical approaches exercised for total repair of TOF, including MV implantation and TAP angioplasty on pulmonary artery (PA) insufficiency and RV failure.
Methods
After review and approval from the Vice-Chancellor’s Office for Research and Technology and the Ethics Committee of Tabriz University of Medical Sciences, Iran, as well as receival of the code of ethics (IR.TBZMED.REC.1397.311), this prospective randomized clinical trial (RCT) was performed. The study was conducted between March 2011 and March 2018, on a total number of 60 patients referred to Shahid Madani Hospital (city of Tabriz) for surgical repair of TOF. The patients were first visited by a pediatric cardiologist and a pediatric cardiac surgeon. Then, echocardiography and routine lab tests were performed to confirm the diagnosis and to determine the inclusion criteria. Upon obtaining informed consent from their parents, the patients who met the inclusion criteria were randomized into two groups. Regarding the informed consent, a written consent form was obtained after explaining the benefits and the complications of each surgical procedure. For the purpose of randomization of patient selection, corresponding to the estimated sample size, 60 cards with two different colors (30 green cards and 30 orange cards) were prepared and then respectively arranged in a black box. After referring the patients for surgery, a card was randomly selected from the box. Accordingly, the patients could be assigned to Group I if the card was green, or to Group II if the card was orange. As well, RVOT resection included reconstruction by TAP in Group I. In Group II, it entailed reconstruction by TAP plus monocusp ventricular outflow patch (MVOP) valve repair. The sample size was set at 60 in accordance with previous studies; it was determined to be 30 patients for each group based on the formula for calculating the sample size with 95% confidence interval (CI) and 80% test power16.
Inclusion criteria
The inclusion criteria were children aged 1-10 years, parental consent to perform elective surgical procedures, normal blood coagulation indices, and moderate pulmonary annular hypoplasia with z-score ranging from -2 to -3.
Exclusion criteria
Patients with mental retardation, pulmonary failure, pulmonary infection or acute respiratory distress syndrome (ARDS), pulmonary atresia, discontinuous pulmonary arteries, as well as those undergoing previous palliative procedures and TOF with an atrioventricular septal defect (AVSD) were excluded from this study.
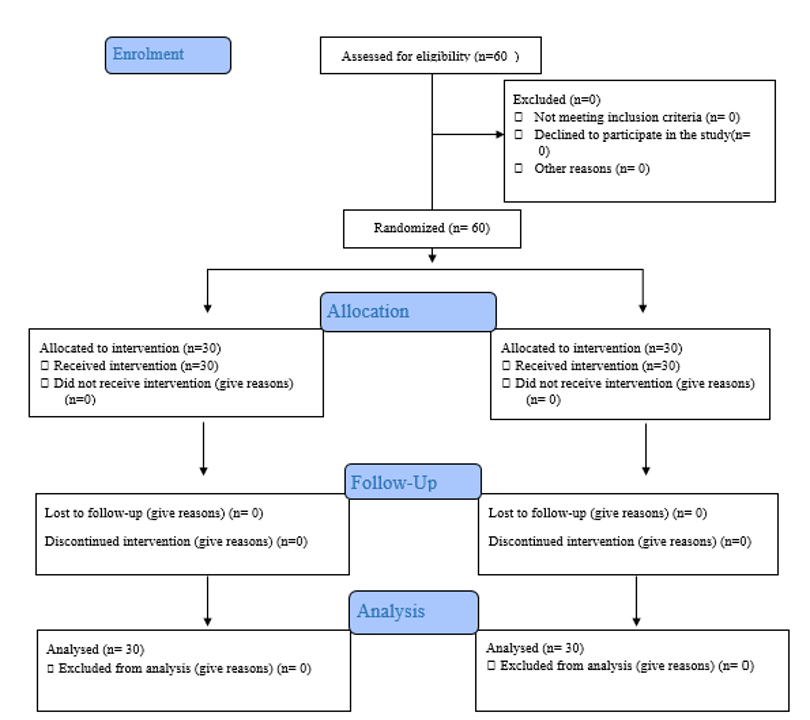
The sample selection process is shown in Figure 1.
In the operating room, the patients were examined by an anesthesiologist who was unaware of their placement in the study groups and who determined the amount of anesthetic drugs. The drugs were injected by the anesthetic nurse and the patients were intubated by the anesthesiologist. The arterial catheter was inserted accordingly into the radial artery by an anesthesiologist and the arterial pressure was monitored. The central venous catheter was also fixed through the internal jugular vein. Cardiac monitoring for all patients began and continued throughout the surgery. Subsequently, the surgical procedure started via midsternotomy incision. Soft tissue incision and bleeding control were further continued with an electrocauter until the heart was exposed and then percutaneous balloon pericardiotomy was done. Following heparin injection, 3 g/kg activated clotting time (ACT) increased up to 450 seconds. At this time, aorto-bicaval cannulation was conducted and CPB was connected to the patients. Cold blood cardioplegia was also completed and it was repeated every 25 min. After that, mild-to-moderate hypothermia induction was performed (28 - 30°C). Then, the band around the superior and inferior vena cava right atriotomy was tightened and RVOT obstruction was resolved by cutting the septal and parietal muscle bundles. Vertical pulmonary arteriotomy was also performed to ensure complete resection of the obstructing bundles. VCD closure was correspondingly fulfilled with a cortex patch using monofilament polypropylene (PP) 0.6 continuous sutures. Ultimately, the right ventricular outlet was reconstructed by two approaches:
Both surgical procedures were fulfilled by the same surgical team. After stabilizing hemodynamic conditions and reversing the effects of heparin with protamine sulphate, the patients were weaned from CPB. Direct measurements of pressure were also used to rule out residual pressure gradients between RV and PA, and the ratio between RV and LV pressures (RVP/LVP ratio) of 30 - 35 mmHg was accepted. The patients were then transferred to the PICU.
Post-operative critical care and follow-up
In the ICU, all the patients were visited twice daily by the anesthesiologist, pediatric cardiac surgeon, and pediatric cardiologist. Full monitoring and mechanical ventilation were also performed. Inotropic and sedative medications were additionally administered if needed. In the absence of hemorragia, and presence of good hemodynamic conditions, improved consciousness and respiratory status, based on the approval of an anesthesiologist, the sedation of the patients was discontinued. Patients were then hyper-oxygenated for 2 min and the endotracheal tube was removed using suction. Oxygen administration was done with mask (7 L/min) until no further need was assured. After extubation, the patients were evaluated using Visual Analogue Scale (VAS) and a diclofenac suppository was used to alleviate pain if needed.
Early post-operative echocardiography (24 h after surgery) was done to evaluate the outcome of surgery and cardiac function of the patients. The data were then recorded for comparisons between both groups. The early outcomes were duration of mechanical ventilation as well as length of PICU stay, mortality, and morbidity. Following the improvement of the general condition of the patients and approval by all three physicians, the patients were transferred to the cardiac surgery ward and discharged after two days. After discharge, all the patients received monthly follow-up twice by a pediatric cardiologist for clinical examinations, chest X-rays, echocardiography, and administration of medications if needed. At the end of the 6th month follow-up, an echocardiography was performed and data were recorded for comparisons between the two study groups.
Study outcomes
PR (none, mild, moderate, and severe), RV failure (fractional area change (FAC) less than 40% and tricuspid annular plane systolic excursion (TAPSE) lower than 10 mm were also considered as more than mild RV failure), ejection fraction (EF), and morbidity were evaluated after 6 months.
Statistical analysis
The data were analyzed using the IBM Statistical Package for Social Sciences (SPSS), version 22. Independent-sample t-test was also employed to compare the means of both quantitative variables. To compare proportions between two qualitative parameters, Chi-square (X2) test was subsequently utilized.
Results
The study population consisted of 39 male (65%) and 21 female (35%) patients. The basic patient characteristics showed no statistically significant differences between the two groups. These comparison data with reference to surgical approaches are shown in Table 1. Furthermore, there was no statistically significant difference in pre-operative PVA z-score (p = 0.263) or PA index (PAI) (p = 0.220) between the two study groups. Additionally, no significant difference was observed in CPB time and aortic cross-clamp time between both groups. However, duration of mechanical ventilation was significantly different between the two groups (the mean number of days for PICU stay in Group I was significantly higher than that in Group II). In general, the mean duration of ICU stay was low in Group I and there was no significant difference between both groups (Table 1).
| Variable | Group I (n=30) | Group II (n=30) | P-value |
|---|---|---|---|
| Gender (male/female) | 16/14 | 23/7 | 0.123 |
| Age | 4 ± 2.259 | 3 ± 2.74 | 0.601 |
| Body weight (kg) | 14.23 ± 3.794 | 12.43 ± 4.014 | 0.223 |
| Body surface area (BSA) (m2) | 0.22 ± 0.04 | 0.21 ± 0.09 | 0.258 |
| PV diameter (mm) | 4.7 ± 1.2 | 4.3 ± 1.2 | 0.594 |
| Z-score of PV diameter | -2.2 ± 0.4 | -2.7 ± 0.7 | 0.263 |
| PAI | 185.9 ± 98.1 | 176.1 ± 89.7 | 0.220 |
| MPA size (mm) | 5.6 ± 2.1 | 5.0 ± 1.01 | 0.285 |
| Pre-operative RVOT pressure gradient (mmHg) | 59.93 ± 7.114 | 62.17 ± 5.052 | 0.328 |
| Post-operative RVOT pressure gradient (mmHg) | 22.12 ± 4.1 | 24 ± 3.08 | 0.147 |
| CPB (min) | 161.67 ± 12.612 | 184 ± 12.594 | 0.805 |
| Aortic cross-clamp time (min) | 121.67 ± 6.315 | 124.70 ± 5.861 | 0.521 |
| Ventilation duration (h) | 49.30 ± 13.134 | 12.7 ± 6.068 | 0.005 |
| Mean ICU stay (days) | 4.97 ± 2.428 | 5.80 ± 4.326 | 0.211 |
Post-operative results and echocardiographic data
The findings revealed that no deaths had occurred during or after surgery until hospitalization. In primary post-operative echocardiography of the patients, PV velocity in Group I and Group II was 2.0 ± 0.5 m/sec and 1.5 ± 0.1 m/sec, respectively; this difference was not statistically significant (p = 0.249). In terms of echocardiography, 24 h after surgery, 18 patients in Group I had severe PR while 12 patients had moderate PR. In Group II, all the patients were also affected with mild PR. Besides, no statistically significant difference was observed in PR grades between the two groups (p = 0.781).
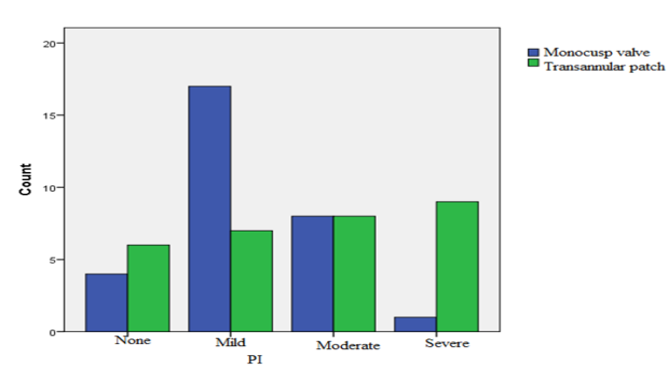
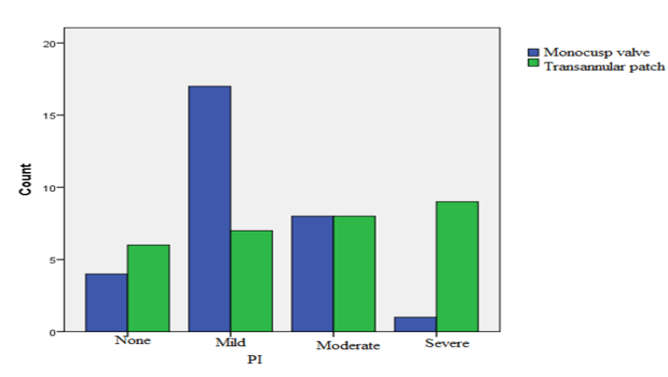
Follow-up echocardiography, performed six months later, demonstrated that in the group with MV implantation, the severity of PA insufficiency was mild in 17 patients (56.7%), moderate in 8 patients (26.7%), and severe in 1 patient (3.3%). Moreover, 4 patients (13.3%) had no PR. In the group undergoing TAP angioplasty, the severity of PA insufficiency was mild in 7 patients (23.3%), moderate in 8 patients (26.7%), and severe in 9 patients (30%). In 6 patients (20%) of this group, no PR was observed. The difference between both groups in terms of severity of PV insufficiency was significant (p = 0.012) (Figure 2). No PV stenosis was reported in patients.
Accordingly, most of the patients with MV angioplasty had moderate PR, while severe PR was very high in the TAP group.
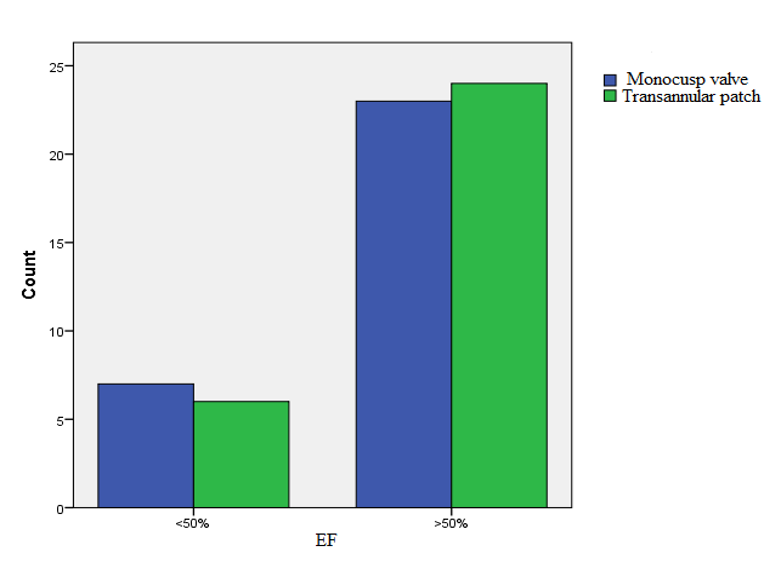
In Group I, 6 patients had an EF of less than 50%, while EF was above 50% in 24 patients. In Group II, 7 patients had an EF of less than 50%, while it was above 50% in 23 patients. A comparison of left ventricular (LV) EF using the Chi-square (X2) test did not show significant differences between both groups (p=0.912) (Figure 3).
As illustrated in Figure 3, EF was low in a small number of patients in both groups; EF in other patients was acceptable.
In Group I, 3 patients had moderate RV failure but 1 patient in Group II suffered from this dysfunction. Moreover, there was no significant difference between both groups (p = 0.510).
Furthermore, 1 and 2 patients lost their lives in Groups I and II, respectively.
No statistically significant differences were observed between the two groups (p = 0.312) (Figure 4). According to Figure 4, few patients in both groups died. There was also no difference between mortality rates in the two groups.
Discussion
The results of this study demonstrated that TOF reconstruction via MV implantation could effectively mitigate PA insufficiency and severity. However, in the short term, it failed to affect RV functioning. Consistent with the results of the present study, other investigations have reported that the use of MV insertion could improve patient outcome by reducing early PR17, 18. However, the major issue causing many complications in TOF patients is increased RV volume overload. If surgical procedures reduce the incidence rate of this complication, it will improve patient outcome.
Different surgical procedures, such as MV reconstruction with autologous pericardium or polytetrafluoroethylene, have thus far been suggested to prevent complications. This corrective surgical technique can moderate RV workload by lowering the degree of PR19, 20. In a study by Jang et al., using a method similar to that utilized in the present study, applying MV implantation with TAP angioplasty compared with TAP alone could delay the grade 3-4 PR. Also, PV stenosis was not observed in patients16. Besides, no PV stenosis was reported at the 6-month follow-up echocardiography in the present study, although this was not a sufficient time for careful examinations and provision of definitive comments. However, the follow-up of the patients in the study by Jang et al. lasted nearly 10 years and, thus, the results from that study could be more reliable16.
Contrary to the results of the present study, other investigations have also revealed that classification and loss of mobility have been observed in a significant number of patients at one year post-surgery16, 21. Additionally, the results of other studies have shown the significant incidence of valvular insufficiency and/or obstruction16, 17, 21, 22. These studies found that MV insertion could cause significant complications and lead to poor patient outcomes. In the post-operative echocardiographic studies, MVs have also exhibited various morphologies. Some have been further attached to PA wall and were non-functional; however, other MVs have been fixated to the middle of RVOT. It was hypothesized that the immobile MVs fixed to the middle of the RVOT might have acted as an anti-regurgitation device, prolonging the interval between initial TOF repair and PR aggravation. However, this mechanism still needs to be elucidated16.
Nevertheless, the main issue has been that synthetic materials for valve replacement cannot grow with children. For that reason, biological materials have been employed to reconstruct monocusp for pulmonary valve in order to preserve growth potential although progressive PR has been highlighted during follow-up periods20, 23. Another study has suggested that it would be better to utilize a standby sparing technique to repair TOF because pulmonary leaflet sparing does not result in significant PR in post-TOF repair and tricuspid repair could protect against future developments of tricuspid regurgitation20.
In this study, mortality rates were low in both groups. It should be noted that RV abnormality is a major element of morbidity and mortality following surgical repair of TOF24. This is justifiable since preserving RV functioning via preventing the right ventriculotomy reduces complications after TOF corrective surgery. The total correction of TOF through a transarterial-transventricular approach for patients aged greater than 6 months has also had a mortality rate of 0 - 2%24.
Conclusion
It was concluded that MV reconstruction of TOF is effective in mitigating PR. However, long-term reviews are needed to evaluate the surgical effects on ventricular dysfunction and to confirm these findings. As limitations, long-term follow-up and surgical complications were not evaluated in this study.
Abbreviations
ACT: Activated clotting time
ARDS: Acute respiratory distress syndrome
AVSD: Atrioventricular septal defect
CPB: Cardiopulmonary bypass
EF: Ejection fraction
FAC: Fractional area change
LV: Left ventricular
LVP: Left ventricular pressure
MPA: Main pulmonary artery
MVOP: Monocusp ventricular outflow patch
PAI: Pulmonary artery index
PI: Pulmonary valve insufficiency
PICU: Pediatric intensive care unit
PR: Pulmonary regurgitation
PV: Pulmonary valve
PVA: Pulmonary valve annulus
RV: Right ventricular
RVH: Right ventricular hypertrophy
RVOT: Right ventricular outflow tract
RVP: Right ventricular pressure
TAPE: Transannular patch enlargement
TAPSE: Tricuspid annular plane systolic excursion
TOF: Tetralogy of fallot
VAS: Visual Analogue Scale
VSD: Ventricular septal defect
Acknowledgments
This article is adopted from the thesis fulfilled by Sayeh Haj Javadi as a Paediatric Resident, and also approved and funded by the Vice-Chancellor’s Office for Research and Technology at Tabriz University of Medical Sciences, Iran. The researchers would like to extend one’s gratitude to the Vice-Chancellor’s Office for Research and Technology at Tabriz University of Medical Sciences and appreciate the staff members of Shahid Madani Hospital for their contribution to this study.
Author’s contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The present study was financially supported by Tabriz University of Medical Sciences, Tabriz, Iran.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Gunduz
E.,
Gorgel
A.,
Dursun
R.,
Durgun
H.M.,
Cil
H.,
Icer
M.,
A Case of Uncorrected Tetralogy of Fallot Undiagnosed Until Adulthood and Presenting With Polycythemia. Cardiology research.
2014;
5
(6)
:
198
.
View Article PubMed Google Scholar -
O'Brien
P.,
Marshall
A.C.,
Tetralogy of Fallot. Circulation.
2014;
130
(4)
:
e26-e9
.
View Article Google Scholar -
V.L. Gott,
C. Walton Lillehei and total correction of tetralogy of Fallot. The Annals of thoracic surgery.
1990;
49
(2)
:
328-332
.
View Article Google Scholar -
Choi
K.H.,
Sung
S.C.,
Kim
H.,
Lee
H.D.,
Ban
G.H.,
Kim
A novel predictive value for the transannular patch enlargement in repair of tetralogy of Fallot. The Annals of thoracic surgery.
2016;
101
(2)
:
703-707
.
View Article PubMed Google Scholar -
Aydın
S.,
Suzan
D.,
Temur
B.,
Kırat
B.,
İyigün
M.,
Demir
I.H.,
The impact of pulmonary valve-sparing techniques on postoperative early and midterm results in tetralogy of Fallot repair. Turkish Journal of Thoracic and Cardiovascular Surgery.
2018;
26
(3)
.
View Article PubMed Google Scholar -
Bigras
J.L.,
Boutin
C.,
McCrindle
B.W.,
Rebeyka
I.M.,
Short-term effect of monocuspid valves on pulmonary insufficiency and clinical outcome after surgical repair of tetralogy of Fallot. The Journal of thoracic and cardiovascular surgery.
1996;
112
(1)
:
33-37
.
View Article Google Scholar -
Mizuno
A.,
Niwa
K.,
The problems related with primary repair for tetralogy of Fallot, especially about transannular patch repair. Translational pediatrics.
2017;
6
(1)
:
8
.
View Article PubMed Google Scholar -
Wankhade
P.R.,
Aggarwal
N.,
Joshi
R.K.,
Agarwal
M.,
Joshi
R.,
Mehta
A.,
Short-term clinical and echocardiographic outcomes after use of polytetrafluoroethylene bicuspid pulmonary valve during the repair of tetralogy of Fallot. Annals of pediatric cardiology.
2019;
12
(1)
:
25
.
View Article PubMed Google Scholar -
Egbe
A.C.,
Mittnacht
A.J.,
Nguyen
K.,
Joashi
U.,
Risk factors for morbidity in infants undergoing tetralogy of fallot repair. Annals of pediatric cardiology.
2014;
7
(1)
:
13
.
View Article PubMed Google Scholar -
Sarris
G.E.,
Comas
J.V.,
Tobota
Z.,
Maruszewski
B.,
Results of reparative surgery for tetralogy of Fallot: data from the European Association for Cardio-Thoracic Surgery Congenital Database. European Journal of Cardio-Thoracic Surgery.
2012;
42
(5)
:
766-774
.
View Article PubMed Google Scholar -
Bouzas
B.,
Kilner
P.J.,
Gatzoulis
M.A.,
Pulmonary regurgitation: not a benign lesion. European heart journal.
2005;
26
(5)
:
433-439
.
View Article PubMed Google Scholar -
Brown
J.W.,
Ruzmetov
M.,
Vijay
P.,
Rodefeld
M.D.,
Turrentine MW. Right ventricular outflow tract reconstruction with a polytetrafluoroethylene monocusp valve: a twelve-year experience. The Journal of thoracic and cardiovascular surgery.
2007;
133
(5)
:
1336-1343
.
View Article PubMed Google Scholar -
Park
C.S.,
Lee
J.R.,
Lim
H.G.,
Kim
W.H.,
Kim
Y.J.,
The long-term result of total repair for tetralogy of Fallot. European Journal of Cardio-Thoracic Surgery.
2010;
38
(3)
:
311-317
.
View Article PubMed Google Scholar -
Kaza
A.K.,
Lim
H.G.,
Dibardino
D.J.,
Bautista-Hernandez
V.,
Robinson
J.,
Allan
C.,
Long-term results of right ventricular outflow tract reconstruction in neonatal cardiac surgery: options and outcomes. The Journal of thoracic and cardiovascular surgery.
2009;
138
(4)
:
911-916
.
View Article PubMed Google Scholar -
Feng
W.,
Yao
J.,
Yang
X.,
Chu
Z.,
Guo
M.,
Wang
L.,
An in vitro study of the influence of monocusp patch size on the hemodynamics for reconstructing right ventricular outflow tract in tetralogy of Fallot. 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE.
2017
.
View Article Google Scholar -
Jang
W.S.,
Cho
J.Y.,
Lee
J.U.,
Lee
Y.,
Surgical results of monocusp implantation with transannular patch angioplasty in tetralogy of fallot repair. The Korean journal of thoracic and cardiovascular surgery.
2016;
49
(5)
:
344
.
View Article PubMed Google Scholar -
Sasson
L.,
Houri
S.,
Sternfeld
A.R.,
Cohen
I.,
Lenczner
O.,
Bove
E.L.,
Right ventricular outflow tract strategies for repair of tetralogy of Fallot: effect of monocusp valve reconstruction. European Journal of Cardio-Thoracic Surgery.
2012;
43
(4)
:
743-751
.
View Article PubMed Google Scholar -
Cowgill
L.D.,
Campbell
D.N.,
Kelminson
L.,
Clarke
D.R.,
Repair of pulmonary valve insufficiency using an autologous monocusp. The Annals of thoracic surgery.
1986;
42
(5)
:
587-589
.
View Article Google Scholar -
He
G.W.,
A new technique of transannular monocusp patch-repair of the right ventricular outflow tract in repair of tetralogy of Fallot. Heart, Lung and Circulation.
2007;
16
(2)
:
107-112
.
View Article PubMed Google Scholar -
Arafat
A.A.,
Elatafy
E.E.,
Elshedoudy
S.,
Zalat
M.,
Abdallah
N.,
Elmahrouk
A.,
Surgical strategies protecting against right ventricular dilatation following tetralogy of Fallot repair. Journal of cardiothoracic surgery.
2018;
13
(1)
:
14
.
View Article PubMed Google Scholar -
Sasikumar
D.,
Sasidharan
B.,
Tharakan
J.A.,
Dharan
B.S.,
Mathew
T.,
Karunakaran
J.,
Early and 1-year outcome and predictors of adverse outcome following monocusp pulmonary valve reconstruction for patients with tetralogy of Fallot: a prospective observational study. Annals of pediatric cardiology.
2014;
7
(1)
:
5
.
View Article PubMed Google Scholar -
Turrentine
M.W.,
McCarthy
R.P.,
Vijay
P.,
Fiore
A.C.,
Brown
J.W.,
Polytetrafluoroethylene monocusp valve technique for right ventricular outflow tract reconstruction. The Annals of thoracic surgery.
2002;
74
(6)
:
2202-2205
.
View Article Google Scholar -
Sen
D.G.,
Najjar
M.,
Yimaz
B.,
Levasseur
S.M.,
Kalessan
B.,
Quaegebeur
J.M.,
Aiming to preserve pulmonary valve function in tetralogy of Fallot repair: comparing a new approach to traditional management. Pediatric cardiology.
2016;
37
(5)
:
818-825
.
View Article PubMed Google Scholar -
Sun
G.,
Wang
X.,
Chen
J.,
Ma
R.,
Li
F.,
Chen
L.,
Primary repair of tetralogy of Fallot in infants: transatrial/transpulmonary or transventricular approach. Asian journal of surgery.
2013;
36
(4)
:
137-143
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 5 (2020)
Page No.: 3799-3806
Published on: 2020-05-26
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5304 times
- Download PDF downloaded - 1113 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress