Abstract
Introduction: The most common malignancy which endangers the quality of life in females is breast cancer. It is also the major public problem in society and despite advances in treatment strategies, it still leads to high mortality. In all breast cancer patients, the first assessment done before surgery is the complete blood count (CBC). There exists a strong relationship between immune response of the body and clinical staging of breast cancer. This study aimed to determine the most reliable hematological markers for the prognosis of breast cancer, and to evaluate the correlation between hematological parameters and disease staging.
Methods: This study was a case control study conducted for a period of 3 years from Jan 2016 to December 2018 in the Department of Pathology (R.L. hospital and Research Center attached to Sri Devaraj Urs medical college, Tamaka, Kolar, Karnataka). The comparison of the hematological parameters was done between the study group (70 cases) and the control group (20 cases). The relation between the hematological parameters and the various stages of breast cancer was analyzed before initiating the treatment.
Results: The majority of the breast cancer patients were of stage 2. The hemoglobin concentration, red blood cell (RBC) count, lymphocyte count, and were reduced in the study group, with a significant difference (p < 0.05) when compared to the controls. Hemoglobin concentration, RBC count, hematocrit, white blood cell (WBC) count, and absolute lymphocyte count were inversely proportional to the stage of breast cancer. The absolute count, ratio (NLR), and platelet-to-lymphocyte ratio (PLR) were directly proportional to the progression of disease.
Conclusion: Hemoglobin, RBC count, WBC count (including differential count), NLR, and PLR are the key hematological indicators which predict the severity and mortality risk of breast carcinoma.
INTRODUCTION
The second most common carcinoma among females is breast cancer, with a prevalence of 99% and also the commonest malignancy which affects half a million women worldwide each year. Annually, in more than 1,000,000 cases of females, the cause of death is breast cancer1.
According to the cancer registry data, the incidence of carcinoma of the breast ranges from 19.3 to 89.7 per 100,000 population (India), with a total of 27.5% cases of cancers in Karnataka2. The prevalence of carcinoma of the cervix in Kolar, Karnataka amounts to 17.6% of all cancer cases in the female population. Squamous cell carcinoma accounts for 80% of cases while adenocarcinoma is relatively rare3. In spite of the improvement in therapies, the mortality rate of cancer is still increasing. Recently, there has been growing interest in investigations on the functions of the immune system in progression and cessation of tumor growth4, 5.
The importance of the immune system in progression of malignancies has been investigated. Hematological parameters like red cell indices, leukocyte count, mean platelet volume (MPV), and platelet counts have shown their prognostic importance in different types of malignancies. The routinely performed investigations before, during and after cancer treatment is the complete blood count (CBC), which roughly estimates the patient’s anemic, nutritional, inflammatory and immunologic status. The neutrophil-to-lymphocyte ratio (NLR), which has been used as a biomarker of inflammation, can also be useful as a prognostic index for various common solid tumors, such as nasopharyngeal cancer, gastric cancer, colorectal carcinoma, breast carcinoma and malignant melanoma. In addition, the NLR has been used as a simple marker of systemic inflammatory response in cancer patients. Similarly, pre-operative platelet-to-lymphocyte ratio (PLR) has also been suggested as a significant factor to predict survival in pancreatic cancer5, 6.
All kinds of diseases and cancers that vary in terms of disease progression have a direct influence on hematological parameters. Breast cancer also shows its effect on hematological parameters and also helps in predicting the outcomes of patients with breast cancer7. Therefore, there is a need for simple, low-cost, reliable and non-invasive prognostic inflammatory biomarkers which will be helpful for clinicians to perform risk evaluation in breast carcinoma patients before or during the treatment process.
This study, therefore, aimed to determine the most reliable hematological markers for the prognosis of breast carcinoma and to evaluate the relationship between hematological parameters and stage of cancer.
MATERIAL AND METHODS
This study was a case control study for a period of 3 years. The study was started after obtaining the approval from the ethics committee (R.L.Jalappa hospital and Research Center attached to Sri Devaraj Urs medical college, Tamaka, Kolar, Karnataka). The study group (70 cases) included female patients who were diagnosed with breast cancer. The demographic data (such as age, menstrual history, parity, history of any previous surgery, clinical presentation, and hematological parameters were obtained from patient medical records. Hematological parameters were compared between the study and the control groups. Analysis of these parameters with the staging of the breast cancer was done prior to the initiation of any treatment, such as surgery, radiotherapy or chemotherapy.
Breast cancer patients were staged clinically according to the tumor, node and metastasis (TNM) classification. Hematological parameters included hemoglobin, red blood cell (RBC) count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and white blood cell (WBC) count. All the parameters were estimated using a 5-part Sysmex automated cell analyzer which works on the principal of volumetric impedance and photometric measurement of light absorbance. Neutrophil-to-lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were also calculated based on the CBC. The NLR was calculated by dividing the absolute neutrophil count and absolute lymphocyte count; cut-off value taken was ≤ 2.21 (area under ROC curve, 0.617; 95% confidence interval). The PLR was calculated by dividing the platelet count and absolute lymphocyte count; the cut-off value was ≤ 143.36 (area under the ROC curve, 0.618; 95% confidence interval)8.
The control group (20 cases) included female patients diagnosed with benign breast disease. The inclusion criteria were patients with breast lumps who had a diagnosis of breast carcinoma by Fine Needle Aspiration Cytology (FNAC) and confirmed by histopathology. The exclusion criteria included breast carcinoma patients who had an infection at the time of sample collection, had undergone neoadjuvant chemotherapy or radiotherapy, and had any other type of malignant lesions.
Statistical analysis
The data obtained were statistically analyzed using the SPSS software version. Mean ± standard deviation was used to express the results; Chi square test was used wherever necessary. The parameters with p < 0.05 were considered to be significant.
Results
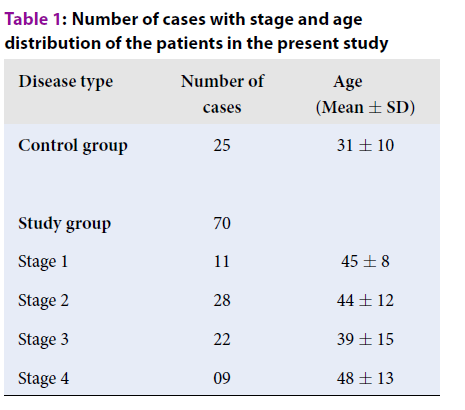
A total of 90 cases of breast disease were analyzed, out of which 70 cases were of breast carcinoma and 20 cases were of benign breast disease. Out of the 70 cases of breast carcinoma, the maximum of cases were seen in stage 2, followed by stage 3, stage 1 and stage 4, respectively (Table 1).
| Disease type | Number of cases | Age (Mean ± SD) |
| Control group | 25 | 31 ± 10 |
| Study group | 70 | |
| Stage 1 | 11 | 45 ± 8 |
| Stage 2 | 28 | 44 ± 12 |
| Stage 3 | 22 | 39 ± 15 |
| Stage 4 | 09 | 48 ± 13 |
All the hematological parameters of the study group were compared with the control group. It was observed that the haemoglobin concentration, RBC count, haematocrit and lymphocyte count were reduced in the study group when compared to the controls, with a significant difference (p < 0.05). However, the RBC indices (MCV, MCH and MCHC), WBC count, neutrophil count and platelet count did not show any significant correlation (Table 2).
The hematological parameters were also compared with the different stages of breast cancer (Table 3). The hemoglobin concentration, RBC, hematocrit, WBC count, and absolute lymphocyte count decreased with disease stage. There was no significant change in RBC indices and absolute neutrophil count. The platelet count was directly proportional to the staging of breast cancer. NLR and PLR showed significant increases in Stage 4 when compared to stage 1.
| Test | Control group | Study group | P-Value |
| Hemoglobin concentration (g/dl) | 11.4 ± 3.8 | 9.8±2.2 | < 0.05 |
| RBC (x 106/ µl) | 4.8 ± 0.6 | 3.98 ± 0.7 | < 0.05 |
| Haematocrit (%) | 35.4 ± 3.6 | 31.8 ± 5.5 | < 0.05 |
| MCV (fL) | 82.2 ± 5 | 80.6 ± 6 | 0.010 |
| MCH (pg) | 26.2 ± 4.2 | 25.6 ± 3.6 | 0.1 |
| MCHC (g/dl) | 33.37 ± 1.02 | 32.85 ± 1.83 | 0.08 |
| WBC (x 103/µl) | 6.35 ± 4.8 | 7.1 ± 1.8 | 0.22 |
| Neutrophil count (%) | 48 ± 7.2 | 65 ± 12.7 | 0.22 |
| Platelet count (x 103/ µl) | 235.7 ± 93.2 | 248 ± 90.8 | 0.99 |
| Lymphocyte count (%) | 35.03 ± 8.29 | 20.91 ± 2.25 | < 0.05 |
| Parameters | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
| Hemoglobin concentration (g/dl) | 10.94 ± 1.27 | 9.42 ± 0.78 | 9.1 ± 1.97 | 8.44 ± 1.28 |
| RBC (x 106/µl) | 4.95 ± 0.90 | 3.5 ± 0.44 | 3.0 ± 0.86 | 2.9 ± 0.8 |
| Hematocrit (%) | 33.8 ± 3.14 | 30.6 ± 7.7 | 28.4 ± 2.0 | 25.6 ± 2.5 |
| MCV (fl) | 82.8 ± 6.7 | 80.8 ± 4.5 | 79.4 ± 3.2 | 78.9 ± 2.5 |
| MCH (pg) | 25.2 ± 4.3 | 26.2 ± 4.2 | 26.78 ± 2.1 | 26.78 ± 1.1 |
| MCHC (g/dl) | 32.1 ± 3.95 | 30.5 ± 2.1 | 32.1 ± 0.8 | 30.8 ± 1.0 |
| WBC (x 103/µl) | 7.7 ± 2.8 | 6.9 ± 1.9 | 5.7 ± 1.7 | 3.9 ± 1.1 |
| Absolute Neutrophil count | 6,502 ± 1226 | 5,323 ± 936 | 6,240 ± 813 | 5,470 ± 6,36 |
| Platelet count (x 103/µl) | 250.2 ± 93.1 | 262.5 ± 85.2 | 287.2 ± 84 | 298.7 ± 90 |
| Absolute Lymphocyte count | 2,825 ± 925 | 2,432 ± 623 | 1,956 ± 840 | 1,784 ± 5.77 |
| NLR | 2.1 ± 1.25 | 2.4 ± 1.08 | 2.8 ± 1.10 | 3.2 ± 1.77 |
| PLR | 90.84 ± 35.33 | 107.60 ± 42.06 | 146.72 ± 40.36 | 150.30 ± 50.36 |
Discussion
Complete blood count (CBC) is a useful basic investigation performed routinely in all cancer patients before, during and after treatment. Various hematological parameters can be measured by this investigation; alterations in any of the parameters further helps to investigate the underlying causes so that treatment can be planned accordingly7, 9. Hence, it is important to also investigate breast cancer patients prior to initiation of any treatments like surgery, radiotherapy and/or chemotherapy. In the current study, we analyzed all the hematological parameters in breast carcinoma patients and found notable variations.
In the present study, the hemoglobin concentration showed a mean of 9.8 g/dl, RBC count of 3.98 x 106/mm3, and hematocrit of 31.8%, when compared to the control group. The majority of the cases had a normocytic normochromic blood picture. Our study correlated significantly with previous studies which showed decreased hemoglobin with normocytic normochromic blood picture. Chronic diseases and cancers can cause decreased production of erythropoietin which in turn leads to decreased production of RBC, resulting in anemia. The other possible causes may be due to low socioeconomic status causing nutritional anemia and metastasis of cancer cells to bone marrow, leading to suppression of all the hematological cell series7, 10. It was also noted that as the stage increased, all these parameters decreased.
The RBC indices did not show any significant correlation with the control group nor also with the different stages of breast carcinoma. The mean total leukocyte count did not show any significant correlation with the control group but as the disease progressed from stage 1 to stage 4, the count decreased. The total leukocyte count is greatly affected by increase or decrease of neutrophils, eosinophils, lymphocytes, monocytes and basophils11. In the present study, the absolute neutrophil count did not show any significant correlation with the control group nor also with staging.
There was a decrease in the absolute lymphocyte count in the present study, with an increase in staging of the breast cancer and severe lymphocytopenia noted in stage 4. These findings are comparable to studies done by Rana APS et al.12 and Parkin DM et al.13, who observed that the decrease in lymphocyte count was proportional to progression of disease. The mechanism of tumor immunity is mainly carried out by CD8+ cytotoxic T-cells which mediate killing of tumor cells and which are capable of destroying tumor cells prior to sensitization. Hence, a decrease in these cells causes suppression of tumor immunity and poor prognosis 12. Higher peripheral blood CD8+ count with higher survival was reported in a study conducted by Lee et al.14.
The mean platelet count, even though it was higher in the study group, did not show any significant correlation with that of the control group; however, the increase in platelet count was directly proportional to the staging of the breast cancer. Systemic inflammation leads to release of several pro-inflammatory mediators, which stimulate megakaryocyte proliferation, such as interleukin (IL)-1, IL-3 and IL-6. Many studies have shown that the higher mortality is associated with elevated platelet count in several cancers. Thus, innate antitumor responses through cell-induced platelet aggregation is modulated negatively by platelets which shields tumor cells from the major histocompatibility complex so as to escape immune surveillance by T cells14, 15.
In the current study, there was an increase in the NLR as the disease progressed. The NLR ratio was found to be higher in patients with axillary lymph node metastasis. Many studies have shown that a pre-operative increase in the NLR ratio suggests poor prognosis of the breast cancer patients; it can be used as an independent prognostic factor in breast carcinoma11, 16.
The process for elevated NLR is explained by relative increase in the neutrophil count and relative decrease in the lymphocyte count, causing the breakdown of balance in the tumour microenvironment. Cellular immune responses decrease as a consequence to lymphocytopenia and there is an increase in the inflammatory response. This increase in inflammation causes an increase in tumor growth, invasion and metastasis11, 15, 16. The usefulness of the NLR in predicting short- and long-term mortality in breast cancer patients was first evaluated by Azab et al.17.
A meta-analysis study showed the association of NLR with the prognosis of the breast cancer (i.e., the higher the values, the shorter the survival time for disease-free survival (DFS) and overall survival (OS), and the poorer the prognosis11, 18.
The present study also demonstrated that increase in pre-operative PLR was significantly associated with the disease progression and axillary lymph node metastasis; these results are in accordance to the study done by Takeuchi H et al.15. The mechanism is due to the platelets which play an important role in tumor progression and to lymphocytes which have a major role in immunosurveillance which suppresses tumor maturation 15. Hence, an elevated PLR in patients with cancer is associated with lymph node metastasis and can be considered as a poor prognostic marker.
Conclusion
Complete blood count impacts the response of cellular immunity on any cancer patient; alterations in any of the hematological parameters shows a high impact on disease progression. Our study showed that the hematological parameters like hemoglobin, RBC count, hematocrit, WBC count, platelet count, absolute neutrophil count, absolute lymphocyte count, NLR and PLR in breast cancer can be a useful guide for disease progression. They can also be advantageous for helping oncologists decide on how to provide further treatment. The increase in NLR and PLR values can be deleterious to cancer patients as those values are associated with poor prognosis and higher mortality.
Abbreviations
CBC: Complete blood count
RBC: Red Blood Cell
WBC: White Blood Cell
MCV: Mean Corpuscular Volume
MCH: Mean Corpuscular Hemoglobin
MCHC: Mean Corpuscular Hemoglobin Concentration
NLR: Neutrophil to Lymphocyte Ratio
PLR: Platelet to Lymphocyte Ratio
DFS: Disease free survival
OS: Overall survival
Acknowledgments
Not applicable.
Author’s contributions
Dr. Shilpa.MD: Concept design, collection of data, review of literature, case collection, statistical analysis, interpretation of the results and preparing manuscript. Dr. Kalyani.R: Concept, editing and review of manuscript. Dr. PN.Sreeramulu: Case collection, editing manuscript.
Funding
Not applicable.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was started after obtaining the approval from the ethics committee (R.L.Jalappa hospital and Research Center attached to Sri Devaraj Urs medical college, Tamaka, Kolar, Karnataka).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Khan
S.,
Khoso
S.A.,
Memon
S.,
Adeel
A.,
Nabi
G.,
Study of some Haematological parameters as Biomarker for breast Cancer population of Sindh. Sindh Univ Res Jour.
2017;
49
(1)
:
23-28
.
-
National Cancer Registry Programme, Indian Council of Medical Research. Leading sites of cancer. In, Consolidated Report of Population Based Cancer Registries 2012- 2014, Incidence and Distribution of Cancer. Bangalore: Coordinating Unit, National Cancer Registry Programme (ICMR).
2016;
14
:
8-30
.
-
Kalyani
R.,
Das
S.,
Singh
M.S.B.,
Kumar
H.M.L.,
Cancer profile in Kolar: A ten years study Indian J Cancer. 2010 ;
47
(2)
:
160-165
.
View Article PubMed Google Scholar -
Smita
S.,
Masamatti
I.,
Vijaya
C.,
Hematological parameters in pre chemotherapy breast cancer patients in a tertiary care centre. Journal of Diagnostic Pathology and Oncology.
2018;
3
(3)
:
237-240
.
View Article Google Scholar -
Arslan
C.,
Aksoy
S.,
Increased mean corpuscular volume of erythrocytes during capecitabine treatment: A simple surrogate marker for clinical response. Tumori.
2011;
97
:
711-716
.
View Article PubMed Google Scholar -
Zhang
P.,
Zong
Y.,
Liu
M.,
Tai
L.,
Prediction of outcome in breast cancer patients using test parameters from complete blood count. Molecular and Clinical Oncology.
2016 ;
(4)
:
918-924
.
View Article PubMed Google Scholar -
Al-arifi
A.A.,
Kumar
A.,
Chigurupati
S.,
Jawed
M.,
Pandurangan
T.,
Pretreatment variations in haematological parameters of breast cancer patients. International Journal of Pharmacy and Pharmaceutical Sciences.
2018;
10
(2)
:
157-161
.
View Article Google Scholar -
Kin
H.Y.,
Kim
T.H.,
Yoon
H.K.,
Lee
A.,
The Role of Neutrophil lymphocyte ratio and platelet lymphocyte ration in predicting neoadjuvant chemptherapy response in breast cancer. Journal of breast cancer.
2019;
22
(3)
:
425-438
.
View Article PubMed Google Scholar -
Olufemi
A.E.,
Saliu
O.A.,
Layiwola
A.M.,
Olayide
A.S.,
Haematological parameters in female breast cancer patients in south western Nigeria. Int J Med Health Sci.
2013;
2
(4)
:
398-401
.
-
Poggiali
E.,
Amicis
M.M.,
Motta
I.,
Anemia of chronic disease: a unique defect of iron recycling for many different chronic diseases. Eur J Intern Med.
2014;
25
:
12-17
.
View Article PubMed Google Scholar -
Chen
L.,
Kong
X.,
Yan
C.,
Fang
Y.,
Wang
J.,
The research progress on the prognostic value of the common haematological parameters in Peripheral venous blood in breast cancer. Onco Targets and Therapy.
2020;
13
:
1397-1411
.
View Article PubMed Google Scholar -
Rana
A.P.S. ,
Kaur
M.,
Zonunsanga
B.,
Puri
A.,
Kuka
A.S.,
Preoperative Peripheral blood count in breast carcinoma: Predictor of prognosis or a routine test. International Journal of Breast cancer.
2015;
2015
:
1-5
.
View Article PubMed Google Scholar -
Parkin
D.M. ,
Bray
E.L.,
Ferlay
J.,
Pisani
P.,
Estimating the world cancer burden. Globocon 2000. International Journal of Cancer.
2001;
94
(2)
:
153-156
.
View Article PubMed Google Scholar -
Lee
Y.T.N.,
xPeripheral lymphocyte count and subpopulations of T and B lymphocytes in benign and malignant diseases. Surgery gynecology and obstretics.
;
144
(3)
:
435-450
.
-
Takeuchi
H.,
Fukuyama
S.,
Kubo
N.,
Kubo
N.,
The Prognostic Signoficance of the preoperative Platelet-Lymphocyte Ratio in Japanese Patients with Localized Breast Cancer. Advances in Breast Cancer Research.
2016;
5
:
49-57
.
View Article Google Scholar -
Elyasinia
F.,
Keramati
M.R.,
Ahmadi
F.,
Neutrophil-Lymphocyte ratio in different stages of breast cancer. Acta Med Iran.
2017;
55
(4)
:
228-232
.
-
Azab
B.,
Bhatt
V.R.,
Phookan
J.,
Murukutla
S.,
Kohn
N.,
Terjanian
T.,
Widmann
W.D.,
Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol.
2012;
19
:
217-224
.
View Article PubMed Google Scholar -
Ethier
J.L.,
Desautels
D.,
Templeton
A.,
Shah
P.S.,
Amir
E.,
Prognostic role of neutrophil to lymphocyte ratio in breast cancer: a systemic review and meta-analysis. Breast Cancer Res.
2017;
19
(1)
:
2
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 8 (2020)
Page No.: 3916-3920
Published on: 2020-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 7672 times
- Download PDF downloaded - 2204 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress


