Abstract
Introduction: Previous studies confirm that there is no anthropometric index of normal Iranian hearts, including diameter of cardiac valve, thickness of myocardial septum and coronary artery variation. The purpose of this study was to assess the anatomical indexes of the human heart and coronary artery in the Iranian population.
Methods: The study was performed with 207 adult human hearts in both sexes (182 male and 25 female), fixed in 10% formaldehyde. Myocardial thickness and diameter of heart valves were measured using a Vernier caliper. Assessments of the coronary artery were carried out after removal of the pericardium.
Results: Analysis of 207 cadaver coronary arteries showed left coronary artery (LCA) dominance type was present in 6.3% of corpus, and balance was present in 4.3% of corpus, while the largest number (89.4%) had right coronary artery (RCA) dominance. The mean values of the morphometric data are as follows: right atrial wall thickness (2.08 +/- 0.04 mm), left atrial wall thickness (2.08 +/- 0.04 mm), interatrial wall thickness (4.92 +/- 0.08 mm), right ventricular wall thickness (3.35 +/- 0.05 mm), left ventricular wall thickness (8.36 +/- 0.13 mm), interventricular wall thickness (12.01 +/- 0.2 mm), diameter of the aorta (23.6 +/- 0.4 mm), diameter of the pulmonary artery (24.94 +/- 0.4 mm), large diameter of the mitral valve (34.16 +/- 0.27 mm), and large diameter of the tricuspid valve (38.8 +/- 0.24 mm).
Conclusion: The anatomical knowledge of cardiac indicators and coronary artery data will be helpful and clinically relevant, especially for cardiac surgeons for coronary artery bypass grafting and coronary arteriography.
Introduction
The heart is a pyramidal organ that pumps blood through the circulatory system. In humans, the heart is located in the thoracic cavity between the lungs. The heart has four chambers that include the left atrium and right atrium, and the left and right ventricles. The right atrium and ventricle are separated by the right atrio-ventricular valve (tricuspid), while the left atrium is associated with the left ventricle through the left atrioventricular valve (mitral). Interatrial, interventricular, and atrioventricular septae separate the four chambers of the heart1, 2, 3. The internal anatomy of each chamber is important for its function4. The right and left coronary arteries (RCA and LCA, respectively) supply the muscle of the heart that normally originates below the junction between the bulbous and the ascending aorta5. The RCA passes vertically between the right atrium and right ventricle in the coronary sulcus. After reaching the inferior border of the heart, it is placed at the diaphragmatic level of the heart. There are several branches of this artery along this course which include: conus artery (CA), atrial rami (AR), sino-atrial artery (SAA), marginal artery (MA), posterior interventricular artery (PIVA), and atrioventricular artery (AV)1, 2. The LCA is thicker than the RCA and passes between the pulmonary trunk and the left auricle, then descends from behind the pulmonary trunk. Eventually, the LCA artery divides into the anterior interventricular (AIVA) and the left circumflex branches (LCX)1, 6.
Variations in the diameter of the heart valves and the coronary arteries are of clinical interest because these findings can be responsive to problems involved in cardiac surgery and angiography. One of these problems is any inconsistency of the size of the artificial valve of the heart or insertion of coronary passages during angiography7, 8. The purpose of this study was to accurately measure heart valves, measure the thickness of atria and ventricles and the septum between them, and to examine the normal variations of coronary arteries using direct dissection for evaluating and determining qualitative changes in the heart. Current indicators include indexes expressed in reference books that are related to Western and European populations, which are consistent with the height, weight, or anthropometric dimensions of these populations. Therefore, this study can be used for guidance by cardiologists, surgeons and medical practitioners involved in the diagnosis and treatment of heart diseases. These findings were observed following dissection from deaths referred to the Department of Forensic Medicine in Isfahan, Iran.
Material — Methods
In the study, 207 hearts from 207 cadavers (182 male and 25 female) were evaluated during gross anatomy examination at Isfahan Medical University (Iran). Postmortem coronary dissection was directly performed in all corpses, with some exclusion criteria such as cardiac disease, endocarditis, and aortic root surgery. Overall autopsies were performed at the Department of Forensic Medicine in Isfahan. These hearts belonged to cases in the age group of 15-50. The hearts were embalmed in 10% formalin.
Determination of the anatomical indexes of the coronary arteries
All hearts were meticulously dissected and first, variations and anomaly of the coronary arteries were noted and photographed along their path. Anatomical indicators of coronary arteries were examined according to the following characteristics:
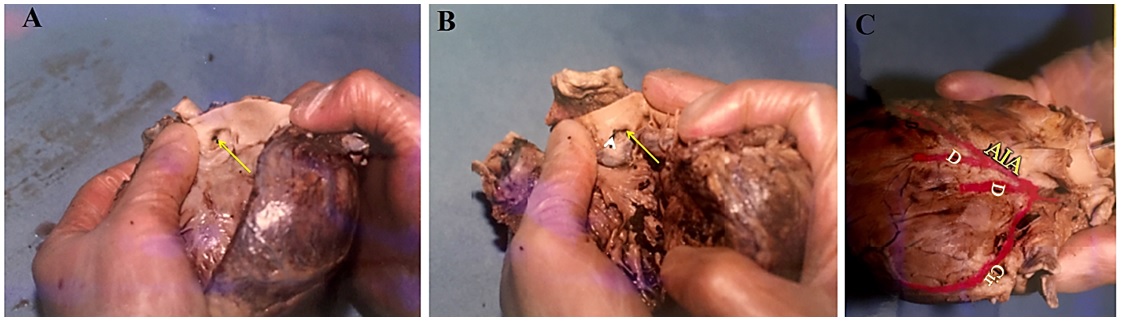
1. Assessment of coronary artery dominance: Right dominant system (A); left dominant system (B); and balance (C) (Figure 1).
2. Assessment of the origin of the conus artery (CA), derived from the trunk of the RCA, or originated from a separate orifice in the anterior aortic sinus (Figure 2).
3. SAA: derived from the right or left coronary artery, or both; if separated from the right coronary artery, first or second part (Figure 3).
4. AV: Whether derived from the right or left coronary artery (Figure 1)
5. Determination of the branches of diagonal artery (Figure 2C)
6. Determination of the length of the left coronary artery (LCX). It is divided into three types: to the left of the heart (short), between the left side of the heart and crux (moderate), and after the crux (high) (Figure 1A and B, Figure 2C).
7. Passing the left and right coronary artery from the crux region (for assessment of coronary artery dominance) (Figure 1B and Figure 2C).
Determination of anatomical indicators in the heart
At this stage, heart indicators such as diameter of the aorta and pulmonary artery, size of the mitral and tricuspid valves, and thickness of the atria and ventricles were examined9, 10, 11.
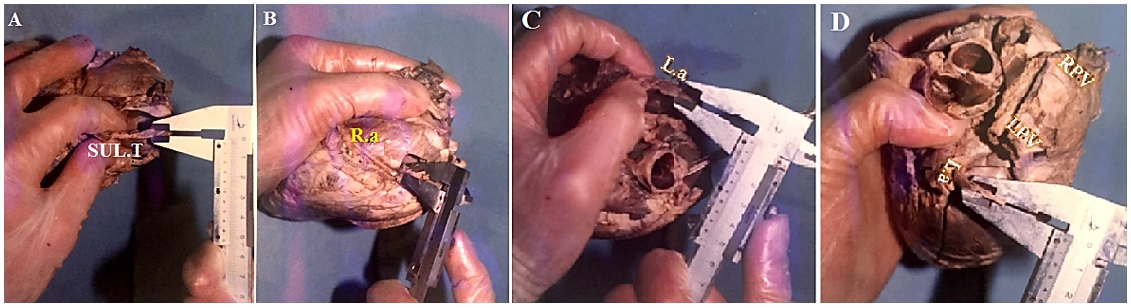
Thickness of the right atrium
Thickness of the right atrium = (Thickness of smooth area*+ Thickness of rough area**)/2
*Thickness of smooth area: the thickness of the right atrium in the sulcus terminalis (Figure 4A).
**Thickness of rough area: the thickness of the right atrium in the pectineal area (Figure 4B)
Thickness of the right atrium
Thickness of the left atrium = (Thickness of smooth area*+ Thickness of rough area**)/2
*Thickness of smooth area: the thickness of the left atrium in the orifice of the pulmonary vein (Figure 4C).
**Thickness of rough area: the thickness of the left atrium in the auricle (Figure 4D).
Wall thickness of the right ventricle (RV): For this purpose, an incision was made between the anterior sinus of the pulmonary artery and parallel to the AIVA up to the apex of the heart; then the average thick and thin areas were measured with a caliper (Figure 5 A and B).
Wall thickness of the left ventricle (LV): To determine the thickness of the right ventricle, an incision was made between the anterior and posterior sinuses of the aortic artery and, similar to the right side, continued to the apex of the heart. The thickness was the average of thin and rough areas (Figure 5 C and D).
The size & diameter of heart valves
The mitral and tricuspid valve annulus were measured in all heart evaluations. For this purpose, a divider, thread and a millimeter scale was used. The diameter of heart valves was taken by keeping a thread at the divider border and the distance of the thread was measured with a millimeter scale. This method is simple and does not require complicated tools.
Determination of the diameter of the aorta and pulmonary artery
Human cadaver hearts were opened longitudinally through the commissure between the left and right coronary cusps. Subsequently, the specimens were mounted flat and the annular circumference of aortic and pulmonary margin were measured near the cusps. Diameter of the valve was assessed through standard circle mathematics, including the equation C = 2nr (Figure 6).
Results
Analysis of the coronary arteries of 207 cadavers that were dissected in our university showed the LCA dominance type was present in 13/207 (6.3%) of corpus, and balance type in 9/207 (4.3%) of corpus, while the largest number 158/207 (89.4%) had the RCA type of coronary dominance (Figure 1).
The normal distribution of coronal arteries was monitored during the dissection. The findings showed that 77/207 (37.2%) of the arteries were separate from the distinct orifice and 128/207 (61.8%) cases were segregated from the right coronary artery; in 2/207 (1%) of cases, this artery was absent.
In studying the origination of the SAA, the data showed that 129/207 (62.3%) of arteries originated from the first part of the RCA, 39/207 (18.8%) from the second part of the RCA, 25/207 (12.1%) from the LCA, 4/207 (1.9%) from the first part of the RCA and the LCA, and 2/207 (1%) from second part of RCA and LCA; in 8/207 (3.9%), this artery was absent.
In addition, the data showed that 186/207 (89.9%) of the AV arteries stemmed from the right coronary arteries, 19/207 (9.2%) stemmed from the left coronary arteries, and in 2/207 (1%), this artery were absent. There were also differences in the number of diagonal arteries in the dissected samples. The frequency of this artery is shown in terms of numbers (Table 1).
| Number of diagonal artery | N | No. of sample (%) |
| 1 | 19 | 9.2 |
| 2 | 73 | 35.3 |
| 3 | 68 | 32.9 |
| 4 | 35 | 16.9 |
| 5 | 10 | 4.8 |
| 6 | 2 | 1 |
| Total | 207 | 100 |
There were differences in the frequency of the lengths of the circumflex artery (LCX) that were measured by coronary dissection (shown in Figure 1 A and B, and Figure 2 C). Thus, according to categorization of this artery, 17.4% (36/207), 56.5% (117/207), and 25.6% (53/207) of the arteries were short, moderate, and tall, respectively. In 0.5% (1/207), the LCX was absent. Also, 4 of the LCX were separated directly from the RCA and placed in the main path after circumvention of the aorta. In studying the passage of the RCA and LCA from the crux region, the findings confirmed that the RCA passed the crux in 163/207 (78.8%) cases, while it was not crossed in 44/207 (21.3%) cases. In the LCA, in 6/207 (2.9%) cases sampled, the arteries crossed the crux area but in 201/207 (97.1%) cases, it did not cross in the majority of specimens. Table 2 shows the results of quantitative assessments, including ventricular wall thickness, atrial wall thickness, interatrial wall thickness, interventricular wall thickness, diameter of the aortic entrance and pulmonary artery, and the large diameter of the mitral valve and tricuspid.
| Quantitative variables | Mean | SEM |
| Right atrium wall thickness | 2.08 | 0.04 |
| Left atrium wall thickness | 2.08 | 0.04 |
| Inter atrial wall thickness | 4.92 | 0.08 |
| Right ventricular wall thickness | 3.35 | 0.05 |
| Left ventricular wall thickness | 8.36 | 0.13 |
| Inter ventricular wall thickness | 12.01 | 0.2 |
| Diameter of the influx of the aorta | 23.6 | 0.4 |
| Diameter of the influx of the pulmonary artery | 24.94 | 0.4 |
| Large diameter of the mitral valve | 34.16 | 0.27 |
| Large diameter of the tricuspid valve | 38.80 | 0.24 |
An investigation of the sex relationship was carried with the above 10 indicators and tests showed no significant differences except in two cases (Figure 7). The two significant cases are as follows:
In addition, age correlation with the top anatomical indicators, except for three, did not show significant differences (p > 0.05). The four significant cases include the following:
With age, the interatrial wall becomes thicker (p < 0.02).
With age, the interventricular wall becomes thicker (p < 0.006).
As age increases, the large diameter of the mitral valve rises (p < 0.01).
As age increases, the large diameter of the tricuspid valve rises (p < 0.002).
Discussion
Measuring myocardial thickness is used for the detection of several cardiovascular diseases11. Mean values of left atrial wall thickness as well as right and left ventricle wall thickness in Gray’s Anatomy were listed as 3 mm, 3 – 5 mm and 8 – 12 mm, respectively1. Our study is similar to this textbook as the mean left atrial wall thickness was 2.08 and the mean right and left ventricle wall thickness measured were 3.35 and 8.36 mm, respectively. We showed that the thickness of both atrium walls was equal, while based on the Gray’s Anatomy textbook, the left atrium has thicker walls than the right atrium. This morphometric difference might be due to race and geography of samples studied. This finding is important because atrial thickness and structural features affect function, such as the electrical wave-dynamic of atrial fibrillation12. In addition, disorders of ventricular function may result from disease states that directly affect the heart muscle because it is necessary to determine normal muscle thickness13, 14.
Matsukubo (1977) and Lang (2015) showed that the right ventricular myocardial thickness was 3-5 mm; their finding are in agreement with those in our present study15, 16. The left ventricular myocardial thickness is transformed in cardiomyopathies, such as hypertrophic cardiomyopathy, and is used to evaluate the presence or absence of disease. Salton led a study on the thickness of left ventricle using cardiac magnetic resonance (CMR), which found that left ventricular myocardial thickness was 9.9 mm in men and 8.7 mm in women, whereas in this current study, we did not find any significant difference in ventricular myocardial thickness between the two sexes. However, in this study, there was a relationship between age and thickness of interatrial and interventricular septum17.
The coronary artery is the main source of blood supply to the heart. Correct interpretation of anatomical variations of the coronary artery is necessary for diagnosis and therapeutic intervention18. This variation is often asymptomatic; however, detection of variation is important for purposes of re-vascularization, angiography, and heart surgery19.
In the present study, 89.4% of cases had RCA dominance, 6.3% had LCA dominance, and 4.3% had balance or co-dominant coronary circulation, consistent with other findings in the literature. The finding of 89.4% of right coronary artery dominance is similar to the incidence found by Kalpana (2003) and Cavalcanti (1995), who observed right dominance in 89% and 88.18% of cases, respectively 20, 21. Many authors have confirmed percentages of right dominance as being between 60% and 80% 22, 23. The dominance pattern is similar between sexes in this study. A previous study showed that although RCA dominance is common in the population, there may not be any association between LCA dominance and heart disease. In another study, it was found that in patients with acute coronary syndrome, left dominance was an independent predictor of enhanced long-term mortality 24, 25.
The coronary dominance pattern can be related to how the posterior interventricular artery (PIVA) separates. In a previous study, 70% of PIVA is a branch of the RCA (known as right dominance), 10% is a branch of the LCA (known as left dominance), and 20% is an anastomosis of the LCA and RCA (known as balance)26. James (1961), and Baroldi and Scomazzoni (1965) showed that in 70% of cases, the RCA passes beyond the crux cordis, while in our study, RCA crossed in 78.8% of cases, which is different from the above studies27, 28. On the other hand, in our study, it was found that the coronal artery was detached in 61.4% of the RCA and in 37.2% of the main trunk. In agreement with our findings, Gray’s textbook mentions the coronal artery in 64% of cases originating from the RCA and 36% separately from the aortic sinus. In another study of 38 dissected adult hearts, only 6 cases (15.8%) showed the coronal artery isolated from the aortic sinus29.
The SAA is not related to coronary arterial dominance30, 31. According to Gray’s, this artery is separate from RCA in 65% of specimens and 35% of the first part of circumflex coronary artery (CCA)1. The SAA pattern in the present study was 81.1% from the RCA, 12.1% from the LCA, 2.9% in both, and absent in 3.9%, which are consistent with those found in the literature. Based on the standard textbook, AV node artery often originates from the RCA (80%). Similarly, incidence of this anomaly was found in 89.9% of our study population. The origin of AV node artery is dependent on coronary arterial dominance30, 31.
Conclusion
The data from this study can provide indicators for an anthropometric index of the heart and coronary artery in the Iranian population. Several studies with large sample sizes (male and female) and advanced techniques (echocardiography, MRI and angiography) are required to augment data about the anatomical features of normal heart and coronary artery.
Abbreviations
AIVA: Anterior interventricular
AR: Atrial rami
AV: Atrioventricular artery
CA: Conus artery
CMR: Cardiac magnetic resonance
LCA: Left coronary artery
LCX: Left circumflex branches
LV: Left ventricle
MA: Marginal artery
PIVA: Posterior interventricular artery
RCA: Right coronary artery
RV: Right ventricle
SAA: Sino-atrial artery
Acknowledgments
This study was supported by Esphahan University of Medical Sciences.
Author’s contributions
Darvishi M, performed the experiments, conceptualization and design the manuscript. Moayeri A explained the data, corrected the manuscript. Authors approved the final manuscript.
Funding
This article has financial support of Ilam University of Medical Sciences.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
This study was accepted by the Ethics Research Committee of Ilam University of Medical Sciences (Ethical code: ir.medilam.rec.1396.147). The research was performed under the laws for human trials.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Gray H. Gray anatomy. 40th ed. London: Churchill. 2008: 992-1010 .
Google Scholar -
Netter F. Human anatomy. 6th ed. US: Saunders. 2014: 205-223 .
Google Scholar -
Snell R. Clinical anatomy. 9th ed. Tokyo. 2012: 40-58 .
Google Scholar -
Libby P, Bonow RO, Mann DL, Zipes DP. Braunwald Heart Diseases a text book of Cardiovascular Medicine 8th ed. Saunders. 2008: 478 .
Google Scholar -
Saidi HS, Olumbe AO, Kalebi A. Anatomy and pathology of coronary artery in adult black Kenyans. East Afr Med J..
2002;
79
(6)
:
323-327
.
View Article PubMed Google Scholar -
Kayrak M, Ulgen MS. Anatomy of coronary arteries. In: Oto A, Ergene O, Kozan O, et al. Textbook of invasive cardiology, 1st ed. Ankara: Hacettepe University Basımevi. 2007: 12-19 .
Google Scholar -
Villa CR, Morales DLS. The Total Artificial Heart in End-Stage Congenital Heart Disease. Front Physiol.
2017;
8
(9)
:
131
.
View Article PubMed Google Scholar -
Altin C, Kanyilmaz S, Koc S, Gursoy YC, Bal U, et al. Coronary anatomy, anatomic variations and anomalies: a retrospective coronary angiography study. Singapore Med J.
2015;
56
(6)
:
339-345
.
PubMed Google Scholar -
Ashalatha PR, Padmini HN. Variations Of The Pulmonary Valve. International Journal of Biomedical Research.
2017;
8
(2)
:
58-63
.
-
Charanya N, Rajathi G, Vishali N. Morphological And Morphometrical Analysis Of Mitral Valve Annulus Of Heart In Human Adult Cadavers. Int J Anat Res.
2017;
5
(3.3)
:
4405-4409
.
View Article Google Scholar -
Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol.
2008;
51
(1)
:
1-11
.
View Article PubMed Google Scholar -
Song J, Wi J, Lee H, Hwang M. Role of atrial wall thickness in wave-dynamics of atrial fibrillation.2017. PLoS ONE.
;
12
(8)
:
e0182174
.
View Article PubMed Google Scholar -
Gupta S, Berry JD, Ayers CR, Peshock RM, Khera A, de Lemos JA, Patel PC, Markham DW, Drazner MH. Left ventricular hypertrophy, aortic wall thickness, and lifetime predicted risk of cardiovascular disease:the Dallas Heart Study. JACC Cardiovasc Imaging.
2010;
3
(6)
:
605-613
.
View Article Google Scholar -
Burke G, Evans G, Riley W, Sharrett A, Howard G. Arterial Wall Thickness Is Associated With Prevalent Cardiovascular Disease in Middle-Aged Adults. Stroke.
1995;
26
(3)
:
386-391
.
View Article PubMed Google Scholar -
Matsukubo H, Matsuura T, Endo N, Asayama J, Watanabe T. Echocardiographic measurement of right ventricular wall thickness. A new application of subxiphoid echocardiography. Circulation.
1977;
56
(2)
:
278-284
.
View Article PubMed Google Scholar -
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging.
2015;
16
(3)
:
233-271
.
View Article PubMed Google Scholar -
Salton J, Chuang M, Donnell C, Kupka M, Larson M. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. 2002;
36
(6)
:
1055-1060
.
View Article Google Scholar -
Reig J, Petit M. Main trunk of the left coronary artery: anatomic study of the parameters of clinical interest. Clin Anat.
2004;
17
:
6-13
.
View Article PubMed Google Scholar -
Fiss DM. Normal coronary anatomy and anatomic variations. Appl Radiology.
2007;
14
(26)
.
-
Kalpana R. A study on principal branches of coronary arteries in humans. J Anat Soc India.
2003;
52
(2)
:
137-140
.
-
Cavalcanti JS, De Lucena Oliveira M, Melo PE, Balaban G, De Andrade Oliveira CL, Lucena OE. Anatomic variations of the coronary arteries. Arq Bras Cardiol.
1995;
65
(6)
:
489-492
.
-
Hutchins GM, Nazarian IH, Bulkley BH. Association of left dominant coronary arterial system with congenital bicuspid aortic valve. Am J Cardiol.
1978;
42
(1)
:
57-59
.
View Article Google Scholar -
Vasheghani-Farahani A, Kassaian SE, Yaminisharif A, Davoodi G, Salarifar M, Amirzadegan A, et al.The association between coronary arterial dominancy and extent of coronary artery disease in angiography and paraclinical studies. Clin Anat.
2008;
21
(6)
:
519-523
.
View Article PubMed Google Scholar -
Ajayi N, Vanker E, Satyapal KS. Coronary artery dominance dependent collateral development in the human heart. Folia Morphol.
2017;
76
(2)
:
191-196
.
View Article PubMed Google Scholar -
Veltman CE, Graaf FR, Schuijf JD, van Werkhoven JM, Jukema JW, Kaufmann PA, Pazhenkottil AP, Kroft LJ, Boersma E, Bax JJ, Schalij MJ, Wall EE. Prognostic value of coronary vessel dominance in relation to significant coronary artery disease determined with non-invasive computed tomography coronary angiography. Eur Heart J.
2012 ;
33
(11)
:
1367-1377
.
View Article PubMed Google Scholar -
Goldberg A, Southern DA, Galbraith PD, Traboulsi M, Knudtson ML, Ghali WA.Coronary dominance and prognosis of patients with acute coronary syndrome. Am Heart J.
2007;
154
(6)
:
1116-1122
.
View Article PubMed Google Scholar -
Baroldi G, Scomazzoni G (1967) Coronary circulation in the normal and pathologic heart. Armed Forces Institute of Pathology, Washington DC, p1-37. .
Google Scholar -
James TN. Anatomy of coronary arteries, New York; Paul B. Hoeber; 1961;12-150. .
Google Scholar -
Vijayamma KN, Ushavathy P. Human coronary arteries: a study based on gross anatomy and coronary cast. J. Evid. Based Med. Healthc.
2018;
5
(6)
:
498-503
.
View Article Google Scholar -
Hutchison MCE. A study on the atrial arteries in man. J Anat.
1978;
125
(1)
:
39-54
.
-
Ramanathan L, Shetty P, Nayak S, Krishnamurthy A, Chettiar G, Chockalingam A. Origin of the sinoatrial and atrioventricular nodal arteries in South Indians: an angiographic study. Arq Bras Cardiol.
2009;
92
(5)
:
314-319
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 9 (2020)
Page No.: 3977-3984
Published on: 2020-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4045 times
- View Article downloaded - 0 times
- Download PDF downloaded - 1010 times
 Biomedpress
Biomedpress