Abstract
Introduction: The purpose of this study was to evaluate and compare the features of the initiation and development of oxidative stress in patients with osteomyelitis and burns.
Methods: We studied the oxidative metabolism of blood of 20 healthy subjects (controls), 15 patients with burns, and 18 patients with chronic osteomyelitis. All patients included in the second group had thermal burns of the I-II-III degree in trunk and limbs on an area of 31 - 80% of the body surface without thermal inhalation trauma. After standard sample preparation, a wide range of parameters of oxidative metabolism was determined in the blood. The intensity of free radical processes in blood plasma and red blood cells, and the total antioxidant activity was evaluated by Fe-induced biochemiluminescence. The concentration of malonic dialdehyde in blood plasma and red blood cells was determined. The level of diene and triene conjugates and Schiff bases was determined spectrophotometrically using reagent kits. The catalase and superoxide dismutase activities in the red blood cells of patients from each of the groups was also determined.
Results: We showed that in osteomyelitis, which is a long-lasting process, changes in the balance of free radical generation and activity of the antioxidant system were compensatory and mostly related to changes in blood plasma. On the contrary, in burn victims, oxidative stress signs had a maladaptive character. They were seen in blood plasma and red blood cells, and accompanied by a pronounced depletion of enzyme antioxidant system reserves.
Conclusion: Our study demonstrate the role of oxidative stress in patients with burns and chronic osteomyelitis, and demonstrate some specific features leading to formation of disease pathology. Such features of oxidative stress may be useful in future design of new approaches to correct the pathology of diseases.
Introduction
It is known that the reactions of free radicals and the opposing activity of a wide range of antioxidant systems are the fundamental basis for various aspects of the functioning of cells and tissues1, 2, 3, 4. In particular, they contribute to the renewal of cellular structures and processes, including biological membranes, energy metabolism, and phagocytic reactions1, 4, 5, 6, 7. All of these processes, combined with oxidative metabolism, can be utilized to disrupt underlying conditions of various pathologies4, 8, 9, 10, 11. This disruptive condition is called oxidative stress, and can be regarded as a complex universal syndrome8, 12, 13, 14, 15. In the laboratory, signs of oxidative stress include a pronounced activation of free radical processes, accompanied by a significant inhibition of the corresponding blood and tissue systems5, 13, 15, 16, 17.
Indeed, despite confirmation in literature of the significant role of oxidative stress in the pathogenesis of various diseases, the specificity of its implementation in a particular pathology is still poorly understood. To a greater extent, this applies to surgical diseases, including traumatological and orthopedic pathologies. A few studies have been recently devoted to this issue. Thus, there is evidence of the involvement of oxidative stress in the pathogenesis of thermal trauma18, 19, 20, 21 and chronic post-traumatic inflammatory processes, such as osteomyelitis22, 23, 24. However, there is no information about the formation and progression of oxidative stress in these diseases. The purpose of the study herein was to conduct a comparative evaluation and analysis of the features of the formation and development of oxidative stress in patients with osteomyelitis and burns.
Material - Methods
Patient and control groups
We studied the oxidative metabolism of blood of 20 healthy subjects (control), 15 patients with burns, and 18 patients with chronic osteomyelitis. All patients included in the second group had thermal burns of the I-II-III degree in the trunk and/or limbs (on an area ranging from 31 - 80% of the body surface); these patients were absent of thermal inhalation trauma. After receiving the study participants’ voluntary informed consent, blood samples from patients of the second and third groups were obtained at admission to the hospital. In patients with burns, blood samples were taken up to 4 days after the moment of injury.
Laboratory assays
After standard sample preparation, a wide range of parameters of oxidative metabolism was determined in the blood. In particular, the intensity of free radical processes in blood plasma and red blood cells, as well as the total antioxidant activity, were evaluated by Fe-induced biochemiluminescence. In addition, the concentration of malonic dialdehyde in blood plasma and red blood cells was determined. The level of diene and triene conjugates, and of Schiff bases, was determined spectrophotometrically using reagent kits. Catalase and superoxide dismutase activities in the red blood cells of patients from each of the designated groups were also determined.
Statistics
The results were processed using the Statistica 6.0 program13. All the data were processed using standard algorithms of descriptive statistics and were presented as Mean±SD. The Student's t-test was used for the detection of statistical differences (p < 0.05 was set as the statistical significance level).
Results
It was discovered that the integral indicator of the intensity of free radical processes in blood plasma and red blood cells (the light sum of Fe-induced biochemiluminescence) showed intergroup differences (Figure 1).
Thus, there is a stimulation of free radical oxidation in the blood plasma of patients with burns and osteomyelitis. This trend is expressed equally (+ 27.8% and + 27.1% relative to practically healthy people, respectively; p < 0.05 for both cases). On the contrary, in the group of patients with osteomyelitis in red blood cells, the indicator values does not differ from that of the control level. However, in severely burned patients, its moderate increase was recorded (+ 8%; p < 0.05 relative to practically healthy people). This indicates the presence of certain features of a shift in oxidative metabolism in the diseases under consideration.
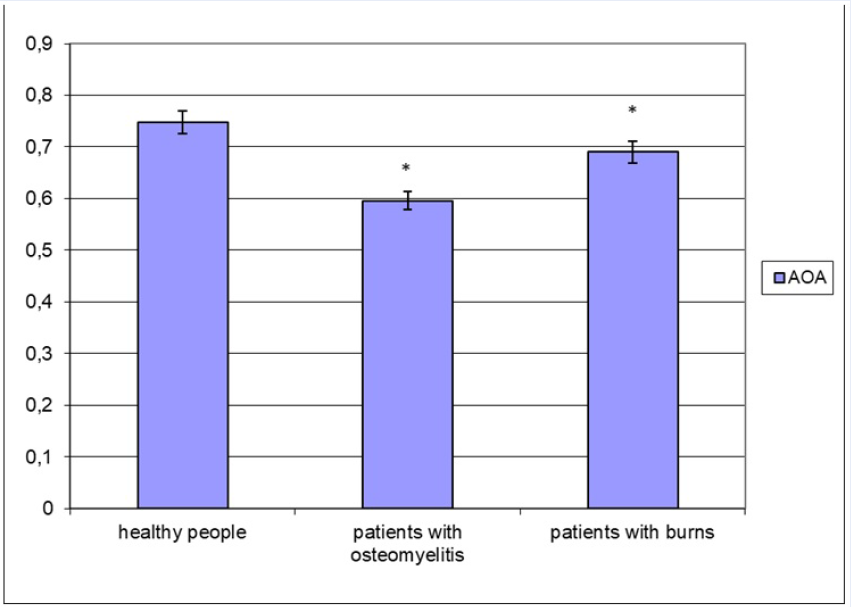
Moreover, the variability of changes is manifested in the total antioxidant activity of blood plasma (Figure 2). It should be noted that both burns and chronic osteomyelitis show a decrease in antioxidant potential of the plasma. Together with an increase in the intensity of free radical processes, this will allow us to record the formation of oxidative stress in both diseases. Simultaneously, the severity of the decrease in the total antioxidant activity is not the same. In particular, in osteomyelitis, the reduction in the indicator level is 20.4%. In contrast, in patients with burns, a decrease in the parameter value was recorded by 7.8% (p < 0.05 for both cases compared to practically healthy people). Simultaneously, the antioxidant activity of blood plasma in severely burned patients is 1.16 times higher than in osteomyelitis (p < 0.05).
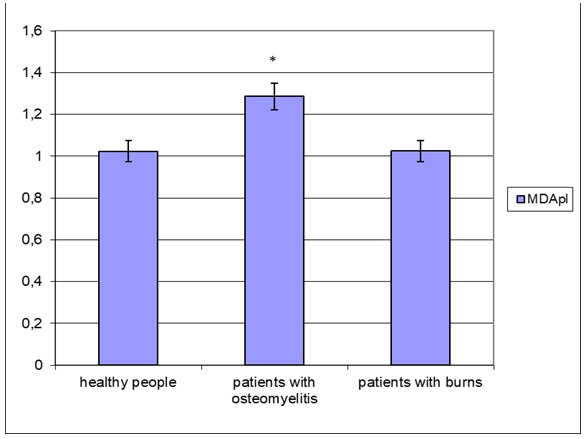
Analysis of the level of malonic dialdehyde in blood plasma and red blood cells also allowed us to verify the specificity of the implementation of oxidative stress in burns and chronic osteomyelitis (Figure 3 and Figure 4). Indeed, in the blood plasma, the concentration of the secondary product of lipid peroxidation increases only in patients with chronic osteomyelitis (by 25.6% relative to the control group; p < 0.05), while in patients with burns, it remains at the level of practically healthy people (Figure 3).
On the contrary, the concentration of malonic dialdehyde in red blood cells increases in representatives of both groups (osteomyelitis and burns), but this trend is expressed differently (Figure 4). A more distinct increase in the red blood cell level of the compound was observed in the burn group (1.54-fold increase compared to healthy group; p < 0.05) than in the chronic osteomyelitis group (+ 39.1%; p < 0.05). It should be noted that the concentration of malonic dialdehyde in burns is significantly higher than in osteomyelitis (by 10.5%; p < 0.05).
According to the assessment results of the level of other lipid peroxidation products, less significant changes were revealed (Figure 5). A moderate increase was found only for diene conjugates, and these changes were expressed almost equally in patients with burns and osteomyelitis (+ 9.7 and + 12.8% relative to the control group, respectively; p < 0.05).
We also evaluated the state of the enzyme antioxidant systems in the patient groups (Figure 6 and Figure 7). It was found that the pathologies had a multidirectional effect on the activity of erythrocyte superoxide dismutase (Figure 6). Thus, in chronic osteomyelitis, moderate activation of the enzyme was noted (by 10.6% relative to practically healthy people; p < 0.05). In patients with burns, there was a significant inhibition of the enzyme (by 20.3%; p < 0.05).
Analysis of red blood cell catalase activity revealed activation only in chronic osteomyelitis (1.25-fold greater than that of the control group; p<0.05), while in severely burned patients, the enzyme’s properties and function remained at the physiological level.
Discussion
It is known that oxidative stress is a universal pathological process (syndrome) that develops in various diseases and conditions8, 9, 10, 11, 12, 13, 14, 15, 16, 17. Simultaneously, there is relatively little information about the specificity of changes in the balance of free radical processes and antioxidant systems in blood and tissues formed within the framework of oxidative stress in specific pathologies1, 4, 5. Therefore, this study attempted to establish the features of specificity for oxidative stress in traumatic pathologies (e.g. burns and chronic osteomyelitis).
The study demonstrated the general signs and features of the implementation of oxidative stress in osteomyelitis and burns. Indeed, the presence of this syndrome in both pathological conditions was confirmed by the detection of characteristic laboratory shifts, including the intensification of free radical oxidation in the blood plasma, as well as suppression of its antioxidant reserves. These results confirm our previously published results18, 19, 20, 21, 22, 23, 24, 25 and those of other authors19, 20, 23, 26, 27. At the same time, the persistent nature of these disorders is emphasized by the detection of increased levels of diene conjugates which increase about equally in patients with burns and osteomyelitis.
On the other hand, there are some features of oxidative stress in the evaluated pathological conditions. Particularly, we suggest that the changes are more related to free radical reactions occurring in the blood plasma in chronic osteomyelitis. This is evidenced by the absence of shifts in Fe-induced biochemiluminescence in the membranes of red blood cells in this pathology and a more pronounced decrease in the total antioxidant activity in blood plasma than in patients with burns. In addition, in osteomyelitis, an increase in the plasma concentration of malonic dialdehyde was observed, which was absent in severely burned patients. Finally, the synchronization of the pathological process in osteomyelitis contributes to the formation of compensatory rearrangements of the enzyme antioxidant system23, 24, as evidenced by a moderate increase in the activity of erythrocyte superoxide dismutase and catalase. We assume that this circumstance is responsible for the lower severity of oxidative metabolism changes in the red blood cells of patients with chronic osteomyelitis.
On the contrary, in burn victims, changes in free radical processes fully affect both blood plasma and red blood cells. This is indicated by a significant increase in the light sum of Fe-induced biochemiluminescence as an integral indicator of the intensity of lipid peroxidation and other free radical reactions in blood plasma and red blood cells. Also, this pathological condition (burn) is characterized by a more pronounced increase in the red blood cell concentration of malonic dialdehyde, in combination with an increase in the level of diene conjugates, than in osteomyelitis. This may indicate that the process is active. In addition, it should be emphasized that an acute thermal burn injury does not involve the inclusion of compensatory mechanisms, in particular, enzyme antioxidant systems18, 20, 21, 26, 28. This leads to a rapid depletion of their reserves during the conditions of intensification of free radical formation. This is indicated by the inhibition of erythrocyte superoxide dismutase activity detected in burns, which ensures the formation of distinct signs of oxidative stress in the red blood cells of patients in this group.
Conclusion
The study herein enabled the verification of oxidative stress in patients with burns and chronic osteomyelitis. The specificity of the formation of the pathological syndrome (oxidative stress) was found. We have shown that in osteomyelitis, since it is a long-lasting process, changes in the balance of free radical generation and the activity of the antioxidant system are compensatory and mostly relate to changes in blood plasma. On the contrary, in burn victims, oxidative stress signs have a maladaptive character. Oxidative stress is seen in blood plasma and red blood cells, accompanied by a pronounced depletion of enzyme antioxidant system reserve. The findings of the oxidative stress features may prove useful in determining a different approach to the correction of pathological conditions, such as burns and osteomyelitis5, 25, 28, 29, 30.
Abbreviations
AOA: antioxidant activity
DC: diene conjugates
TC: triene conjugates
ShB: Schiff bases
Author's contributions
A.K.M., V.I.Z. and A.G.S. contributed to the conceptualization and design of the study, the analysis and interpretation of data. A.S.P. carried out the selection and supervision of patients. K.L.B. and A.G.S. performed laboratory tests. They were drafting the article and revising the article critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This article had no financial support of this faculty.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The study was approved by Local Ethic Committee of Privolzhsky Research Medical University (no. 2 from 18/02/2018).
Consent for publication
The authors hereby consents that the Publisher publishes the Work.
Competing interests
The authors declare that they have no competing interests.
References
-
Azzi A, Davies KJ, Kelly F. Free radical biology - terminology and critical thinking. FEBS Lett. 2004;558(1-3):3-6.
.
View Article Google Scholar -
Bartz RR, Piantadosi CA Clinical review: oxygen as a signaling molecule. Crit. Care 2010;14(5):234.
.
View Article PubMed Google Scholar -
Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1-4. doi: 10.1016/j.freeradbiomed.2013.02.011.
.
View Article PubMed Google Scholar -
Pomatto LCD, Davies KJA. Adaptive homeostasis and the free radical theory of ageing. Free Radic Biol Med. 2018;124:420-430.
.
View Article PubMed Google Scholar -
Martusevich AK, Soloveva AG, Martusevich AA, Karuzin KA, Peretyagin SP. Bioradical homeostasis as new complex parameters of different biological systems. Journal of Integrated OMICS. 2020;10(2):29-32.
.
View Article Google Scholar -
Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R491-511.
.
View Article PubMed Google Scholar -
Tyurina YY, Tyurin VA, Anthonymuthu T, Amoscato AA, Sparvero LJ, Nesterova AM, Baynard ML, Sun W, He R, Khaitovich P, Vladimirov YA, Gabrilovich DI, Bayır H, Kagan VE. Redox lipidomics technology: Looking for a needle in a haystack. Chem Phys Lipids. 2019;221:93-107.
.
View Article PubMed Google Scholar -
Djordjevic A Mladenovic,
Dietary Restriction and Oxidative Stress: Friends or Enemies? Antioxid Redox Signal. 2021;34(5):421-438.
.
View Article PubMed Google Scholar -
Singh A, Kukreti R, Saso L, Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24(8):1583.
.
View Article PubMed Google Scholar -
Taysi S, Tascan AS, Ugur MG, Demir M. Radicals, Oxidative/Nitrosative Stress and Preeclampsia. Mini Rev Med Chem. 2019;19(3):178-193.
.
View Article PubMed Google Scholar -
Tejero J, Shiva S, Gladwin MT. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol Rev. 2019;99(1):311-379.
.
View Article PubMed Google Scholar -
Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury. Part I: basic mechanisms and in vivo monitoring of ROS. Circulation 2003;21:1912-6.
.
View Article PubMed Google Scholar -
Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849-68.
.
View Article PubMed Google Scholar -
Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27-33.
.
View Article PubMed Google Scholar -
Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180-3.
.
View Article PubMed Google Scholar -
Guo Q, Li F, Duan Y, Wen C, Wang W, Zhang L, Huang R, Yin Y. Oxidative stress, nutritional antioxidants and beyond. Sci China Life Sci. 2020;63(6):866-874.
.
View Article PubMed Google Scholar -
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264.
.
View Article PubMed Google Scholar -
Martusevich AK, Dmitrochenkov AV, Razumovsky AV, Galova EA. Possibilities of monitoring the physical and chemical properties of biological fluids in combustiology. Medicine (Russia). 2018;1:149-160.
.
View Article Google Scholar -
Mutwedu VB, Nyongesa AW, Oduma JA, Kitaa JM, Mbaria JM. Thermal stress causes oxidative stress and physiological changes in female rabbits. J Therm Biol. 2021;95:102780.
.
View Article PubMed Google Scholar -
Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6-17.
.
View Article PubMed Google Scholar -
Roshangar L, Soleimani Rad J, Kheirjou R, Reza Ranjkesh M, Ferdowsi Khosroshahi A. Skin Burns: Review of Molecular Mechanisms and Therapeutic Approaches. Wounds. 2019;31(12):308-315.
.
-
Chen DS, Cao JG, Zhu B, Wang ZL, Wang TF, Tang JJ. Baicalin Attenuates Joint Pain and Muscle Dysfunction by Inhibiting Muscular Oxidative Stress in an Experimental Osteoarthritis Rat Model. Arch Immunol Ther Exp (Warsz). 2018;66(6):453-461.
.
View Article PubMed Google Scholar -
Grbic R, Miric DJ, Kisic B, Popovic L, Nestorovic V, Vasic A. Sequential analysis of oxidative stress markers and vitamin C status in acute bacterial osteomyelitis. Mediators Inflamm. 2014;2014:975061.
.
View Article PubMed Google Scholar -
Jyoti A, Singh S, Mukhopadhyay B, Gavel R, Mishra SP. Free radicals and antioxidant status in chronic osteomyelitis patients: a case control study. J Clin Diagn Res. 2015;9(4):BC08-10.
.
View Article PubMed Google Scholar -
Martusevich AK, Razumovsky AV, Soloveva AG, Ezhevskaya AA, Peretyiagin SP. The study of postburn metabolic rehabilitation with natural NO donor. Asian Journal of Biochemical and Pharmaceutical Research. 2016;6(3):14-21.
.
-
Masch JL, Bhutiani N, Bozeman MC. Feeding During Resuscitation After Burn Injury. Nutr Clin Pract. 2019 Oct;34(5):666-671.
.
View Article PubMed Google Scholar -
Moreira E, Burghi G, Manzanares W. Update on metabolism and nutrition therapy in critically ill burn patients. Med Intensiva. 2018;42(5):306-316.
.
View Article PubMed Google Scholar -
Martusevich AK, Peretyagin SP, Ruchin MV, Struchkov AA. Ozone therapy in patients with burn disease. J. Biomedical Science and Engineering. 2018;11(2):27-35.
.
View Article Google Scholar -
Vassalle C, Maltinti M, Sabatino L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules. 2020;25(11):2653.
.
View Article Google Scholar -
Yang B, Chen Y, Shi J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem Rev. 2019;119(8):4881-4985.
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 8 No 3 (2021)
Page No.: 4286-4293
Published on: 2021-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6117 times
- Download PDF downloaded - 1865 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress