Abstract
Background: Coronavirus disease affects mainly the respiratory system. Other systems, including blood, are also affected. Blood cell abnormalities have varied between studies. The majority of patients present with platelet abnormalities.
Methods: This was a laboratory observation study. All cases positive for the coronavirus disease 2019 (COVID-19) by reverse transcriptase — polymerase chain reaction (RT-PCR) test during the study period were considered for inclusion. Platelet index data were captured from an automated hematology analyzer: platelet count, mean platelet volume (MPV), platelet distribution width (PDW), plateletcrit (PCT), and platelet–large cell ratio (P-LCR). Platelet lymphocyte ratio (PLR), platelet neutrophil ratio (PNR), and platelet monocyte ratio (PMR) were calculated. The cases were classified into two groups: moderate and severe. The difference in alteration of platelet parameters between moderate and severe COVID-19 cases was analyzed using SPSS 22 version software. A p-value of < 0.05 was considered statistically significant.
Results: Most cases (44.9%) were in the age group of 41 – 60 years. The male-to-female ratio was 1.9:1. Moderate cases comprised 53.4%, and 46.6% of cases were severe. The association of PLR and PNR between moderate and severe cases was statistically significant. PLR was higher in severe cases than moderate cases, whereas PNR was higher in moderate cases than severe cases.
Conclusions: Studying platelet index profiles in COVID-19 patients can improve our limited knowledge of the disease progression regarding platelet parameters. PLR and PNR are the more reliable platelet parameters in managing COVID-19 patients, which help predict the prognosis and aid in improving therapeutic options for severe cases.
Introduction
Coronavirus disease affects mainly the respiratory system, but other systems can also be affected, including hematologic parameters. Blood cell abnormalities have varied between studies; most cases have shown decreased lymphocytes and neutrophilia, and a few have shown thrombocytopenia1. The mechanisms by which coronavirus affects the hematopoietic system are not clear.
The majority of coronavirus infection patients present with platelet abnormalities. A possible mechanism may be a decrease in platelet production due to bone marrow aplasia, because of either the effect of cytokines or the direct effect of coronavirus on the bone marrow2.
This study reviews the changed platelet indices in coronavirus disease 2019 (COVID-19) disease. Interpreting these changes in patients infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) could contribute to timely diagnosis and predict the prognosis.
Methods
This laboratory-based observational study was performed over six months, from October 2020 to March 2021, at the Department of Pathology in a tertiary healthcare center in South India. The study obtained ethical clearance from the institute ethics committee. The objectives were to assess the profile of platelet parameters in COVID-19-positive cases and analyze the difference in the profile of platelet parameters between moderate and severe COVID-19-positive cases. All cases testing positive for COVID-19 by reverse transcriptase–polymerase chain reaction (RT-PCR) test were included. Cases with incomplete clinical details were excluded.
The sociodemographic data of all the cases were captured from the hospital record section. The platelet index data were collected from the hematology section at the Department of Pathology. The platelet parameters captured by automated hematology analyzer were platelet count, mean platelet volume (MPV), platelet distribution width (PDW), plateletcrit (PCT), and platelet–large cell ratio (P-LCR). Platelet lymphocyte ratio (PLR), platelet neutrophil ratio (PNR), and platelet monocyte ratio (PMR) were calculated using the platelet count, absolute lymphocyte count, absolute neutrophil count, and absolute monocyte count obtained with the automated hematology analyzer. The cases were classified into two groups: moderate and severe. Patients with ≥ 90% oxygen saturation were defined as moderate COVID-19, and patients with room air oxygen saturation < 90% were defined as severe COVID-193, 4. The changes in platelet indices were analyzed for all cases. The difference in alteration of platelet parameters was analyzed between the moderate and severe COVID-19 groups.
The data were entered into a Microsoft Excel datasheet and analyzed using SPSS version 22 software (IBM SPSS Statistics, Somers, NY, USA). Categorical data were represented as frequencies and proportions. Continuous data were represented as mean and standard deviation. An independent t-test was used as a test of significance to identify the mean difference between two quantitative variables. A p-value of < 0.05 was considered statistically significant after assuming all the rules of statistical tests.
| Age of subjects | Frequency | Percentage |
|---|---|---|
| < 20 yrs | 21 | 5.1% |
| 21 - 40 yrs | 94 | 22.9% |
| 41 - 60 yrs | 184 | 44.9% |
| 61 - 80 yrs | 99 | 24.1% |
| 81 - 100 yrs | 12 | 2.9% |
| Total | 410 | 100.0% |
| Sex | Frequency | Percentage |
|---|---|---|
| Female | 141 | 34.4% |
| Male | 269 | 65.6% |
| Total | 410 | 100% |
| COVID-19 | Subjects | Percentage |
|---|---|---|
| Moderate | 219 | 53.4% |
| Severe | 191 | 46.6% |
| Total | 410 | 100.0 |
| Platelet parameters | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| PDW (%) | 0.1030 | 23.3000 | 12.095861 | 2.6740860 |
| MPV (fl) | 7.8000 | 17.2000 | 10.488049 | 1.0881575 |
| P-LCR (%) | 8.2000 | 58.3000 | 28.357561 | 8.1372129 |
| PCT (%) | 0.0200 | 30.0000 | 0.347805 | 1.5639965 |
| PLR | 10.8100 | 1304.3000 | 219.609537 | 176.2386736 |
| PMR | 30.7600 | 4081.8000 | 502.860683 | 435.9225552 |
| PNR | 0.9400 | 187.8000 | 43.369976 | 33.8220995 |
| Platelet count (x 10 9 /L) | 16 | 612 | 241.48 | 101.406 |
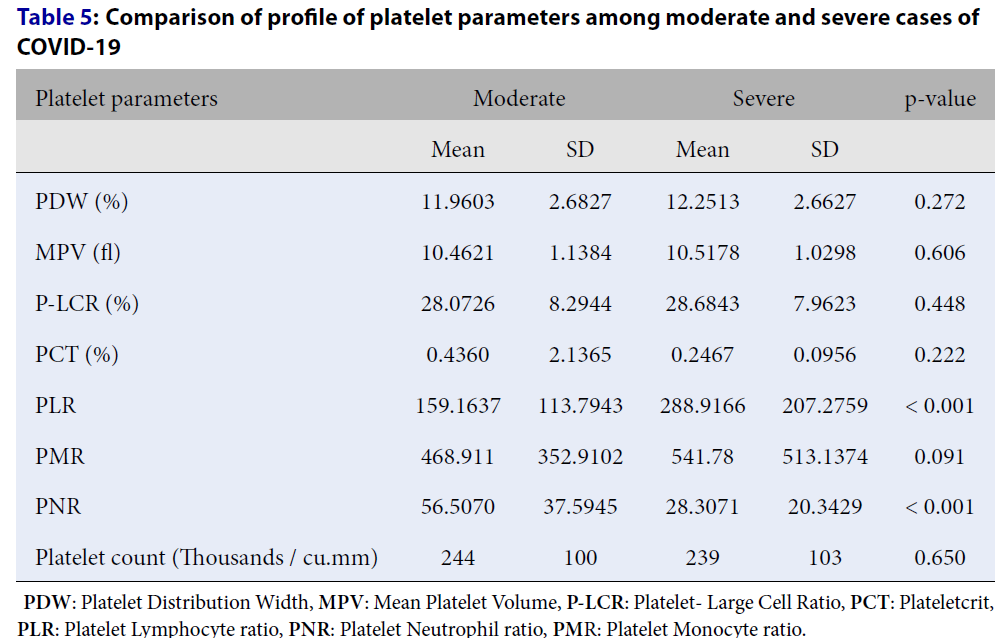
| Platelet parameters | Moderate | Severe | p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| PDW (%) | 11.9603 | 2.6827 | 12.2513 | 2.6627 | 0.272 |
| MPV (fl) | 10.4621 | 1.1384 | 10.5178 | 1.0298 | 0.606 |
| P-LCR (%) | 28.0726 | 8.2944 | 28.6843 | 7.9623 | 0.448 |
| PCT (%) | 0.4360 | 2.1365 | 0.2467 | 0.0956 | 0.222 |
| PLR | 159.1637 | 113.7943 | 288.9166 | 207.2759 | < 0.001 |
| PMR | 468.911 | 352.9102 | 541.78 | 513.1374 | 0.091 |
| PNR | 56.5070 | 37.5945 | 28.3071 | 20.3429 | < 0.001 |
| Platelet count (Thousands / cu.mm) | 244 | 100 | 239 | 103 | 0.650 |
Results
A total of 410 cases were analyzed. Most cases (44.9%) were in the age group of 41 – 60 years (Table 1). Most were men, constituting 269 cases (65.6%), and women represented 141 cases (34.4%). The male-to-female ratio was 1.9:1 (Table 2). Moderate cases comprised 53.4%, and 46.6% of cases were severe (Table 3).
The mean PDW was 12.09 ± 2.67%. Among moderate cases, the mean PDW was 11.96 ± 2.68%, and it was 12.25 ± 2.66% in severe cases. The difference in PDW between moderate and severe cases was not statistically significant (p = 0.272; Table 4, Table 5).
The mean MPV was 10.48 ± 1.08 fL. Among moderate and severe cases, the mean MPV was 10.4 ± 1.13 and 10.51 ± 1.02 fL, respectively. The difference in MPV between moderate and severe cases was not statistically significant (p = 0.606; Table 4, Table 5).
The mean P-LCR was 28.35 ± 8.13%. Among moderate cases, the mean P-LCR was 28.07 ± 8.29%, and it was 28.68 ± 7.96% among severe cases. The difference in P-LCR between moderate and severe cases was not statistically significant (p = 0.448; Table 4, Table 5).
The mean PCT was 0.34 ± 1.56%. Among moderate cases, the mean PCT was 0.43 ± 2.13%, and among severe cases, it was 0.24 ± 0.09%. The difference of PCT between moderate and severe cases was not statistically significant (p-value 0.222) (Table 4, Table 5).
The mean PLR was 219.60 ± 176.23%. Among moderate cases, the mean PLR was 159.1 ± 113.7%, and among severe cases, it was 288.9 ± 207.2%. The difference in PLR between moderate and severe cases was statistically significant (p < 0.001; Table 4, Table 5).
The mean PMR was 502.86 ± 435.92%. Among moderate cases, the mean PMR was 468.9 ± 352.9%, and it was 541.7 ± 513.1% among severe cases. The difference in PMR between moderate and severe cases was not statistically significant (p = 0.091; Table 4, Table 5).
The mean PNR was 43.36 ± 33.82%. Among moderate cases, the mean PNR was 56.50 ± 37.59%, and among severe cases, it was 28.3 ± 20.3%. The difference in PLR between moderate and severe cases was statistically significant (p < 0.001; Table 4, Table 5).
The mean platelet count was 241.48 ± 101.40 x109/L. Among moderate cases, the mean platelet count was 244 ± 100 x109/L, and among severe cases, it was 239 ± 103 x109/L. The difference in platelet count between moderate and severe cases was not statistically significant (p = 0.650; Table 4, Table 5).
Discussion
COVID-19 is a pandemic viral disease caused by the novel coronavirus SARS-CoV-2. Hematologic abnormalities are observed in COVID-19 patients. We aimed to analyze platelet parameters in moderate and severe COVID-19 cases.
In this study, male patients were predominantly affected, accounting for 269 cases (65.6%); women constituted 141 cases (35.4%). This was similar to the study by Ozcelik et al.3. In our study, most cases were in the age group of 41 – 60 years (n = 184, 44.9%). The cases were further subdivided into moderate and severe cases. More cases were categorized as moderate (n = 219, 53.4%).
PDW is a measurement of platelet anisocytosis, determined by calculating individual platelet volumes, and the normal range is 10.0 – 17.9%5. In the present study, the mean PDW was within the normal range for both moderate and severe cases. Ozcelik et al. reported higher PDW levels in COVID-19-positive patients3. In COVID-19, the PDW value is expected to be higher due to a cytokine storm leading to platelet production and destruction mechanisms. PDW determines the platelet size distribution range. A high PDW level indicates the destruction of immature platelets6. In our study, the normal PDW is probably due to a normal platelet count in the majority of cases (only 20% had thrombocytopenia).
MPV is a measure of the average size of platelets, and the normal range is 7.5 – 11.5 fL3. The mean MPV was within the normal range for both categories of cases in the present study. MPV is considered an inflammatory marker, and it determines the size of platelets7. MPV levels are increased in several diseases but reduced in viral diseases8, 9. The reason for decreased MPV is the destruction of young platelets at the site of inflammation. Ozcelik et al. reported statistically significantly (p = 0.027) decreased MPV in COVID-19 cases3. Guçlu et al. reported a decrease in MPV at follow-up of COVID-19 patients, associated with significant mortality10. Our study did not include follow-up of cases.
P-LCR is defined as the percentage of platelets that surpass the normal platelet volume value of 12 fL in the total platelet count. The P-LCR normal range is 15 – 35%3. The mean P-LCR was slightly increased in both moderate and severe COVID-19 cases in the present study, but this was not statistically significant. P-LCR is a platelet index used to predict inflammation in numerous diseases, such as autoimmune diseases. Due to the expeditious inflammatory processes in COVID-19, severe patients have demonstrated increased P-LCR6, 11.
PCT is a measure of total platelet mass, with a normal range of 0.20 – 0.36%12. PCT in the present study was slightly higher in both moderate and severe COVID-19 cases, but this was not statistically significant. He et al. found that PCT was not significant in COVID-19 cases (p = 0.0545)11.
PLR is defined as the ratio of platelet to absolute lymphocyte counts. The normal range for PLR is 36.63 – 149.13%13. The mean PLR was increased in severe COVID-19 cases compared to moderate cases, and this was statistically significant (p < 0.001). PLR has been advocated as a novel biomarker for predicting the prognosis and severity of COVID-1914. Simadibrata et al. found increased PLR in severe COVID-19 cases. The underlying pathogenesis of high PLR levels in severe COVID-19 cases is not clearly understood. A possible explanation is a decline in absolute lymphocyte count, which may be greater than the decline in platelet count, leading to increased PLR in severe cases15.
PMR is defined as the ratio of platelet to absolute monocyte counts. The normal range is 7 — 22%. In the current study, the mean PMR ratio was altered in both moderate and severe cases, indicating significant changes in monocytes and platelet counts, but the difference was not statistically significant between the groups. PMR parameters in COVID-19 cases have not been assessed by any study in the English literature to the best of our knowledge.
PNR is defined as the ratio of platelet to absolute neutrophil counts. The p-value was < 0.001 between moderate and severe cases in the present study. PNR was more increased in the moderate COVID-19 cases than severe cases. This could be due to a cytokine storm in severe cases of COVID-19, which causes recruitment of many neutrophils, neutrophilia, and increased absolute neutrophil count, resulting in decreased PNR. Hence, PNR may be a prognostic marker for COVID-19 cases. PNR has also been used as a prognostic marker in acute ischemic stroke16. However, no published literature has been found regarding PNR in COVID-19.
In this study, thrombocytopenia was observed in 20% of cases, the majority of which were severe. However, no statistically significant difference existed between moderate and severe cases in platelet count. Evidence suggests that platelet and platelet indices have a role in prothrombotic responses to viral infections17. Some studies have reported a relationship between thrombocytopenia and severity of COVID-19, describing the mechanisms as suppression of bone marrow due to COVID-19 infection resulting in a reduction in platelet production, platelet destruction because of increased immune and inflammatory responses, and platelet consumption due to microthrombus formation in the lungs and other organs18, 19. Endothelial cell damage causes activation and aggregation of platelets, leading to thrombus formation and causing depletion of platelets and eventually megakaryocytes, ultimately resulting in reduced platelet production19. Platelets also express surface receptors that enable the entry and binding of numerous viruses20.
This study’s limitations were that it was unicentric with a relatively small sample size, and the cases were not followed up. However, estimation of platelet parameters is cheap and widely available, and some parameters can differentiate between moderate and severe COVID-19 cases. COVID-19 is a novel infection, so it is essential to recognize the biomarkers that may help predict the severity of the disease and aid as a prognostic factor. Research with a larger population is required to validate the findings of our study so that the information can be used for better care of COVID-19 patients.
Conclusions
Studying platelet index profiles in COVID-19 patients can improve our limited knowledge of disease progression regarding platelet parameters, help predict prognosis, and possibly aid in improving therapeutic options for severe cases. PLR and PNR are the more reliable platelet parameters in managing COVID-19 patients.
Abbreviations
COVID-19: Corona virus disease 2019
MPV: Mean Platelet Volume
PCT: Plateletcrit
PDW: Platelet Distribution Width
P-LCR: Platelet- Large Cell Ratio
PLR: Platelet Lymphocyte ratio
PMR: Platelet Monocyte ratio
PNR: Platelet Neutrophil ratio
RT-PCR: Reverse transcriptase polymerase chain reaction
Acknowledgments
None.
Author’s contributions
Nikhil Chaudhary: Data collection, review of literature, writing manuscript; Kalyani Raju: Concept, review of literature, manuscript editing, manuscript revision; Prabhakar Kamarthi: Data collection, manuscript editing
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Chen
N.,
Zhou
M.,
Dong
X.,
Qu
J.,
Gong
F.,
Han
Y.,
Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet.
2020;
395
(10223)
:
507-13
.
View Article PubMed Google Scholar -
Chan
J.F.,
Yuan
S.,
Kok
K.H.,
To
K.K.,
Chu
H.,
Yang
J.,
A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet.
2020;
395
(10223)
:
514-23
.
View Article PubMed Google Scholar -
Ozcelik
N.,
Ozyurt
S.,
Yilmaz Kara
B.,
Gumus
A.,
Sahin
U.,
The value of the platelet count and platelet indices in differentiation of COVID-19 and influenza pneumonia. Journal of Medical Virology.
2021;
93
(4)
:
2221-6
.
View Article PubMed Google Scholar -
Guan
Wj.,
Ni
Zy.,
Hu
Y.,
Liang
Wh.,
Ou
Cq.,
He
Jx.,
Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med.
2020;
382
(18)
:
1708-1720
.
View Article PubMed Google Scholar -
Chang
D.,
Lin
M.,
Wei
L.,
Xie
L.,
Zhu
G.,
Dela Cruz
C.S.,
Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. Journal of the American Medical Association.
2020;
323
(11)
:
1092-3
.
View Article PubMed Google Scholar -
Gao
Y.,
Li
Y.,
Yu
X.,
Guo
S.,
Ji
X.,
Sun
T.,
The impact of various platelet indices as prognostic markers of septic shock. PLoS One.
2014;
9
(8)
:
e103761
.
View Article PubMed Google Scholar -
Mete
E.,
Akelma
A.Z.,
Cizmeci
M.N.,
Bozkaya
D.,
Kanburoglu
M.K.,
Decreased mean platelet volume in children with acute rotavirus gastroenteritis. Platelets.
2014;
25
(1)
:
51-4
.
View Article PubMed Google Scholar -
Wang
X.,
Meng
H.,
Xu
L.,
Chen
Z.,
Shi
D.,
Lv
D.,
Mean platelet volume as an inflammatory marker in patients with severe periodontitis. Platelets.
2015;
26
(1)
:
67-71
.
View Article PubMed Google Scholar -
Karagöz
E.,
Ulçay
A.,
Turhan
V.,
Mean platelet volume and red blood cell distribution width in prognosis of chronic hepatitis B. Wiener Klinische Wochenschrift.
2014;
126
(7-8)
:
250-1
.
View Article PubMed Google Scholar -
Guclu
E.,
Kocayigit
H.,
Okan
H.D.,
Erkorkmaz
U.,
Yurumez
Y.,
Yaylacı
S.,
Effect of COVID-19 on platelet count and its indices. Rev Assoc Med Bras.
2020;
66
(8)
:
1122-7
.
View Article PubMed Google Scholar -
He
J.,
Wei
Y.,
Chen
J.,
Chen
F.,
Gao
W.,
Lu
X.,
Dynamic trajectory of platelet-related indicators and survival of severe COVID-19 patients. Critical Care (London, England).
2020;
24
(1)
:
607-11
.
View Article PubMed Google Scholar -
Kadhem
S.J.,
Raheem
A.H.,
Aljumaily
H.S.,
Shammari
A.A.,
Humairi
A.K.,
Baay
A.,
Platelets Profile Changes in Patients with COVID 19. Sys Rev Pharm.
2020;
11
:
569-74
.
-
Liu
X.,
Zhang
R.,
He
G.,
Hematological findings in coronavirus disease 2019: indications of progression of disease. Annals of Hematology.
2020;
99
(7)
:
1421-8
.
View Article PubMed Google Scholar -
Simadibrata
D.M.,
Pandhita
B.A.,
Ananta
M.E.,
Platelet to lymphocyte ratio; A novel biomarker to predict the severity of COVID-19 patients: A systematic review and meta-analysis. The Journal of the Intensive Care Society.
2020;
:
1751143720969587
.
View Article Google Scholar -
Henry
B.M.,
de Oliveira
M.H.,
Benoit
S.,
Plebani
M.,
Lippi
G.,
Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine.
2020;
58
(7)
:
1021-8
.
View Article PubMed Google Scholar -
Jin
P.,
Li
X.,
Chen
J.,
Zhang
Z.,
Hu
W.,
Chen
L.,
Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. Journal of Clinical Neuroscience.
2019;
63
:
110-5
.
View Article PubMed Google Scholar -
Hottz
E.D.,
Bozza
F.A.,
Bozza
P.T.,
Platelets in immune response to virus and immunopathology of viral infections. Frontiers in Medicine.
2018;
5
:
121
.
View Article PubMed Google Scholar -
Xu
P.,
Zhou
Q.,
Xu
J.,
Mechanism of thrombocytopenia in COVID-19 patients. Annals of Hematology.
2020;
99
(6)
:
1205-8
.
View Article PubMed Google Scholar -
Guo
L.,
Rondina
M.T.,
The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Frontiers in Immunology.
2019;
10
:
2204
.
View Article PubMed Google Scholar -
Chabert
A.,
Hamzeh-Cognasse
H.,
Pozzetto
B.,
Cognasse
F.,
Schattner
M.,
Gomez
R.M.,
Human platelets and their capacity of binding viruses: meaning and challenges?. BMC Immunology.
2015;
16
(1)
:
26
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 10 (2021)
Page No.: 4649-4654
Published on: 2021-10-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5369 times
- PDF downloaded - 1044 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress


