Abstract
Background: The role of pro and anti-inflammatory cytokines in autoimmune and inflammatory disorders has always been discussed; several studies have assayed serum levels of Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), and Interleukin-10 (IL-10) in alopecia areata (AA) patients. Determining the cytokine profile of AA patients will help us understand the role of the immune system in the pathogenesis of AA. Due to a lack of comprehensive studies in this regard, we have performed this meta-analysis to evaluate previously mentioned cytokines in AA patients.
Methods: We explored PubMed, Scopus, web of science, and ScienceDirect databases for research without a limited start date until May 2021. A number of 1098 studies were found in the initial database search and reference lists of relevant studies; 16 studies were finally included in the meta-analysis. Differences in the levels of IL-6, TNF-α, and IL-10 in sera of controls and patients were pooled as standardized mean differences. A random-effects model was used in this study. Begg's and Egger's tests were carried out to investigate publication bias.
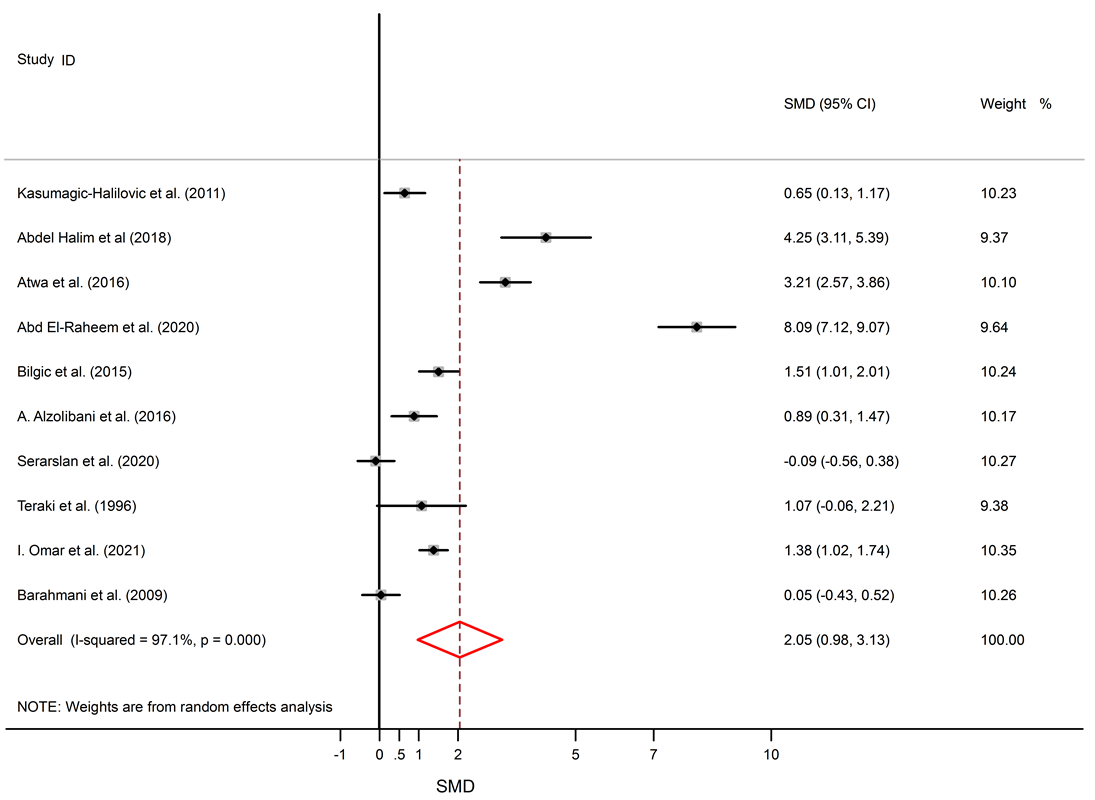
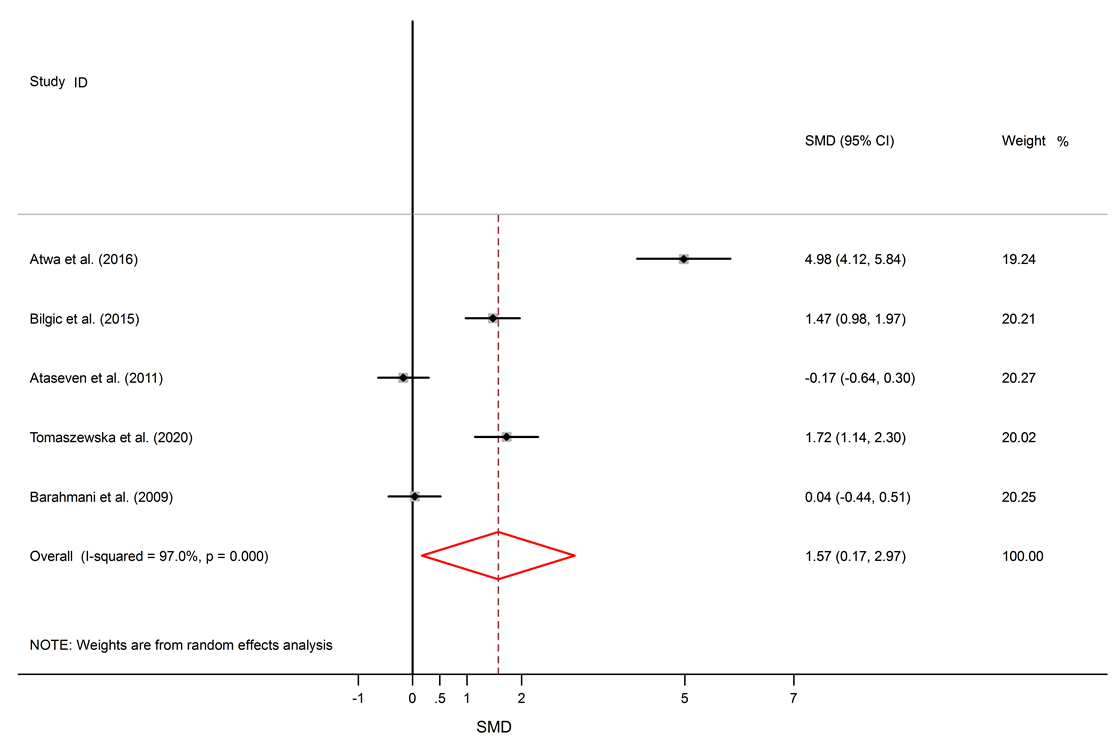
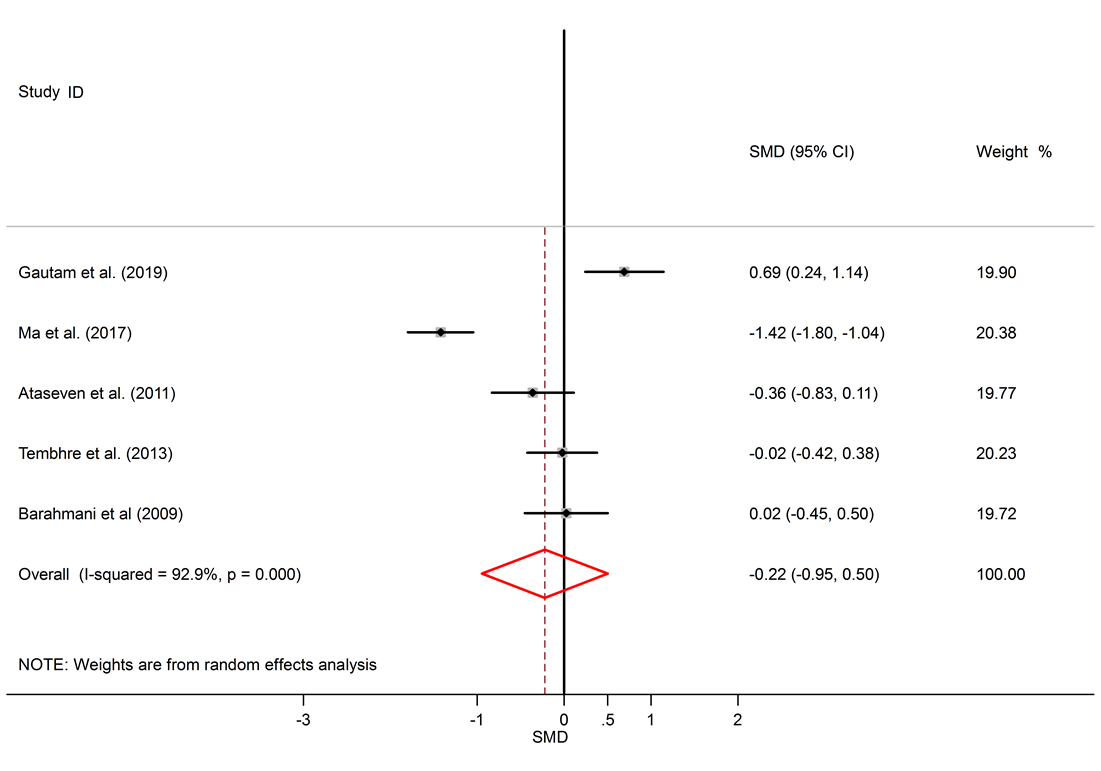
Results: 16 studies were included. This study found significantly elevated levels of IL-6 in AA patients compared to healthy subjects (SMD = 1.57, 95% CI 0.17 to 2.97). The levels of TNF-α were also significantly higher in the serum of AA patients (SMD = 2.05, 95% CI 0.98 to 3.13). Though IL-10 levels were lower in serum of AA patients, this difference was not significant (SMD = -0.22, 95% CI -0.95 to 0.50).
Conclusion: According to the crucial role of cytokines in autoimmunity, alternation in the serum levels of cytokines in AA patients was not unexpected; our study shows that cytokines might have an essential role in the pathogenesis of AA, though further studies are needed to clarify the exact role of cytokines in the emergence and persistence of AA.
INTRODUCTION
Alopecia areata (AA) is an autoimmune, inflammatory, and nonscarring disease that leads to hair loss on the body or scalp. It has two main subgroups: Alopecia Totalis (AT) and Alopecia Universalis (AU). The former includes those patients with a total absence of terminal scalp hair, and the latter include those patients with complete loss of terminal scalp and body hair1. Few studies of prevalence and incidence have reported a 0.1 – 0.2% incidence with a lifetime risk of 1.7% equally in men and women. Furthermore, about 2% of new dermatology patients in the United States and the United Kingdom suffer from AA1, 2.
AA mostly shows itself through a sudden hair loss in well-demarcated sites. The area is commonly an oval or round patch of alopecia, and it can be numerous or isolated. Previous documents noted that though the most common complication of this disease is the involvement of the scalp hair, it may affect body hair such as underarm hair, beard, eyelashes, eyebrows, and pubic hair2, 3, 4, 5.
Circumstances such as infection and tissue injury can result in inflammation6. The inflammatory state can represent itself by elevated inflammatory cytokines such as IL-6, TNFα, and CRP, and a decrease of i-inflammatory cytokines such as IL-107, 8. An inappropriate autoimmune response may stem from a chronic inflammatory condition9. It has been demonstrated that defects in cytokines and their signaling can lead to autoimmune diseases10. It is confirmed that cytokines play a crucial role in the CD4+ T cell-mediated inflammatory response in AA11.
IL-6 and TNF-α are two inflammatory cytokines that play an important role in different inflammatory disorders like rheumatoid arthritis and psoriasis12, 13. Recent studies on the serum levels of these two inflammatory cytokines in AA patients have shown contradictory results11, 14, 15. Furthermore, recent studies on the serum levels of IL-10 in AA patients have shown paradoxical results, including both increasing and decreasing16, 17.
Several studies have explored the links between AA and IL-6, IL-10 and AA and TNF-α11, 16, 18. Due to inconsistent results and the lack of comprehensive studies, we performed a meta-analysis to determine the role of cytokines in AA and to provide more evidence for future related studies.
METHODS
Search method
PubMed, Scopus, Web of Science, and ScienceDirect were searched following the Preferred Reporting of Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines19. We used the following keywords: ‘Alopecia areata’ and ‘Interleukin-10’ or
‘IL-10’, ‘Interleukin-6’ or ‘IL-6’, ‘TNF-α’ or ‘tumor necrosis factor-alpha'. The reference lists of relevant studies were also reviewed. No restriction for the time was applied, and we searched databases from inception to May 2021.
Exclusion and inclusion criteria
Only English articles were included in this study. The title and abstract of identified documents were reviewed to select relevant articles, and in case of doubt, full texts were reviewed. Duplicate studies and irrelevant studies such as review articles, case series, case reports, animal studies, genetic studies, and studies that did not report sufficient data were excluded.
Data Extraction and Quality Assessment
We extracted the following information from the included studies: first author, publication year, country, disease type, measurement method, age of patients and healthy controls, and serum levels of IL-10, IL-6, or TNF-α. If the mean and SD values were not mentioned in the text of manuscripts, we estimated them from given data such as median, range, and IQR.
The risk of bias was evaluated by NIH Quality Assessment Tools20. Quality assessment was performed by two authors.
Statistical analysis
Random-effects meta-analysis was used to estimate standardized mean difference (SMD). The publication bias was assessed using Begg’s and Egger’s tests21. The heterogeneity of results between studies was checked by I2 statistic22, STATA/SE 11.0, and was utilized for statistical analysis (Stata-Corp, College Station, TX, USA).
| First author | Year | Country | Subgroups/Severity of disease | Number of cases/ healthy control | IL-6 levels in patients | IL-6 levels in healthy controls | IL-6 measurement | Age of cases/controls |
|---|---|---|---|---|---|---|---|---|
| Atwa et al . 23 | 2016 | Egypt | Single patch AA, Multiple AA, Ophiasis, AU, AT | 47/40 | 17.18 ± 3.08 | 4.59 ± 1.66 | ELISA | 22.68 ± 8.62 / 23.22 ± 8.95 |
| Bilgic et al . 11 | 2015 | Turkey | Mild, Moderate, Severe | 40/40 | 25.70 ± 9.7 | 11.80 ± 9.2 | ELISA | 28.7 ± 7.9 / 30.5 ± 8.4 |
| Ataseven et al . 14 | 2011 | Turkey | Not mentioned | 43/30 | 1.36 ± 0.39 | 1.44 ± 0.56 | ELISA | 23.42 ± 11.41 / 26.73 ± 4.70 |
| Tomaszewska et al . 24 | 2020 | Poland | Patchy, and Sever | 33/30 | 121.38 ± 31.01 | 75.26 ± 21.15 | ELISA | 18.64 ± 8.56 / 19.95 ± 13.08 |
| Barahmani et al . 18 | 2009 | USA | Transient AA, Persistent AA, AT or AU | 269/18 | AAP: 1.20* AAT: 1.50* AT ⁄ AU: 1.30* | 0.69* | Mutli-analyte proteome array | 39 ± 18.8 / 44 ± 13.6 |
| First author | Year | Country | Subgroups/Severity of disease | Number of cases/ healthy control | IL-10 levels in patients | IL-10 levels in healthy controls | IL-10 measurement | Age of cases/controls |
|---|---|---|---|---|---|---|---|---|
| Gautam et al . 16 | 2019 | India | Localized and Extensive AA | 40/40 | 3.144* | 1.050* | Sandwich ELISA | 29.4 ± 8.3 / 22.6 ± 3.8 |
| Loh et al . 25 | 2018 | Korea | Patchy, AT/AU, ADTA, Ophiasis | 55/15 | 4.54 ± 0.001 | 3.25 ± 0.071 | ELISA | 38.16 / 41.17 |
| Ma et al . 17 | 2017 | china | Severe AA in active or stable phase | 100/50 | 21.20 | ELISA | Active phase: 41.2 ± 13.5, Stable phase: 40.9 ± 14.4/43.5 ± 16.1 | |
| Ataseven et al . 14 | 2011 | Turkey | Not mentioned | 43/30 | 5.94 ± 1.17 | 6.32 ± 0.85 | ELISA | 23.42 ± 11.41 / 26.73 ± 4.70 |
| Tembhre et al. 26 | 2013 | India | Extensive AA,AT,AU | 51/45 | 9.47* | 10.47* | ELISA | 24.19 ± 6.69 / 26.42 ± 4.35 |
| Barahmani et al . 18 | 2009 | USA | Transient AA, Persistent AA, AT or AU | 269/18 | AAP: 0.85* AAT: 0.90* AT ⁄ AU: 0.85* | 0.69* | Mutli-analyte proteome array | 39 ± 18.8 / 44 ± 13.6 |
| First author | Year | Country | Subgroups/Severity of disease | Number of cases/ healthy control | TNF-alpha levels in patients | TNF –alpha levels in healthy controls | TNF –alpha measurement | Age of cases/controls |
|---|---|---|---|---|---|---|---|---|
| Kasumagic-Halilovic et al . 27 | 2011 | Bosnia and Herzegovina | LAA,AT/AU | 60/20 | 10.31 ± 1.20 | 9.59 ± 0.75 | ELISA | 35.6 / 32.6* |
| Abdel Halim et al. 28 | 2018 | Egypt | Multiple patches | 20/20 | 102.44 ± 16.16 | 39.03 ± 13.57 | Elisa | 28.4 ± 11.408 / 29.02 ± 13.57 |
| Atwa et al . 23 | 2016 | Egypt | Single patch, Multiple patches, Ophiasis ,AT ,AU | 47/40 | 19.94 ± 3.59 | 9.95 ± 2.42 | ELISA | 22.68 ± 8.62 / 23.22 ± 8.95 |
| Abd El-Raheem et al . 29 | 2020 | Egypt | Mild, Moderate, Severe | 75/75 | 8.8* | 1.4* | ELISA | 24.8 ± 18.6 / 26.3 ± 4.328 |
| Bilgic et al. 11 | 2015 | Turkey | Mild, Moderate, Severe | 40/40 | 20.60 ± 6.20 | 10.20 ± 7.50 | ELISA | 28.7 ± 7.9 / 30.5 ± 8.4 |
| Loh et al . 25 | 2018 | Korea | Patchy, AT /AU, ADTA), Ophiasis | 55/15 | 12.76 ± 0.003 | 3.33 ± 0.011 | ELISA | 38.16 / 41.17 |
| A. Alzolibani et al . 30 | 2016 | Saudi Arabia | Patchy persistent | 25/26 | 27.70 | 14.70 | Sandwich ELISA | 30.2 ± 8.43 / 32.3 ± 10.8 |
| Serarslan et al. 15 | 2020 | turkey | Patch pattern, Patch and ophiasis pattern, AT,AU | 36/34 | 113.68 ± 106.34 | 121.15 ± 55.31 | sandwich ELISA | 31.33 ± 9.47 / 30.5 ± 9.11 |
| Teraki et al . 31 | 1996 | Japan | Localized form | 7/7 | 8.30 ± 0.90 | 7.60 ± 0.20 | Radioimmunoassay kit | 24 / 21 |
| I. Omar et al . 32 | 2021 | Egypt | Patchy, AU, AT, Ophiasis | 72/75 | 5.3* | 3.9* | ELISA | Adults: 37.6 ± 12, Children: 11 ± 3.3/ Adults: 37.4 ± 13.7, Children: 12.9 ± 2.8 |
| Barahmani et al . 18 | 2009 | USA | Transient AA, Persistent AA, AT or AU | 269/18 | AAP: 3.58* AAT: 3.06* AT ⁄ AU: 2.97* | 2.46* | Mutli-analyte proteome array | 39 ± 18.8 / 44 ± 13.6 |
Results
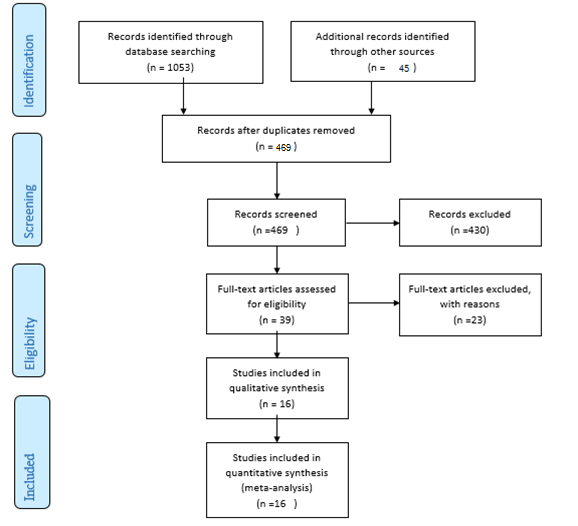
A total of 1098 articles were found in the aforementioned databases. 629 duplicate papers were excluded. 453 studies were excluded after a review of the titles and abstracts. Finally, 16 studies were selected to be involved. The detailed search process is shown in Figure 1. Extracted data for IL-6, IL-10, and TNF-α are reported in Table 1, Table 2 and Table 3, respectively. The levels of IL-10 in the serum of healthy subjects were higher than AA patients, but the difference was not significant (SMD = -0.22, 95% CI -0.95 to 0.50).
Among six studies that report IL-10 levels in the sera of AA patients, one study shows reduced levels of IL-10 in AA patients compared to healthy controls17, one study shows a raised level16, and four studies show no significant difference14, 18, 26, 25. There was a significant elevation of IL-6 in the serum of AA patients compared to healthy controls (SMD = 1.57, 95% CI 0.17 to 2.97).
Among 5 studies that describe IL-6 levels, three studies show elevated levels of IL-6 in AA patients11, 23, 24, and two studies show no significant difference between AA patients and healthy subjects14, 18. Significantly elevated levels of TNF-α in the serum of AA patients compared to healthy subjects were found (SMD = 2.05, 95% CI 0.98 to 3.13).
Among 11 studies that describe TNF-α levels in AA patients and healthy controls, eight studies describe higher levels of TNF-α in AA patients11, 25, 23, 27, 28, 29, 30, 32, and no significant difference was seen in three studies15, 18, 31. Forest plots of TNF-α, IL-6, and IL-10 are shown in Figure 2, Figure 3 and Figure 4, respectively. The p-values of Begg’s tests (p = 0.014) and Egger’s tests (p = 0.021) for IL-6 indicate evidence of publication bias.
Discussion
Alopecia areata is an autoimmune disorder that causes small patches of baldness on the scalp or/and body, and it is linked to some coexisting autoimmune disorders such as rheumatoid arthritis, vitiligo, and psoriasis33, 34. The pathogenesis of AA is not completely understood. It seems that it is associated with a complex interplay between genetic susceptibility and immune system function33, 35. The abnormal levels of pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokine (IL-10) in AA were confirmed by previous documents11, 16. The present meta-analysis discovers a significant link between AA and the inflammatory markers, with SMD for TNF-α (SMD = 2.05, 95% CI 0.98 to 3.13), followed by IL-6 (SMD = 1.57, 95% CI 0.17 to 2.97) and then IL-10 (SMD = -0.22, 95% CI -0.95 to 0.50).
The molecular mechanisms responsible for these alterations in immune function need to be explored more fully36. The objective of this meta-analysis was to collate data from published literature to evaluate the serum TNF-α, IL-6, and IL-10 levels in patients with AA.
TNF-α is manufactured by a wide range of cells, including immune and non-immune cells, and is considered to be a pro-inflammatory cytokine that has an essential role in the pathogenesis of many inflammatory diseases such as arthritis, rheumatoid, and psoriasis37, 38, 39. Our meta-analysis shows increased serum levels of TNF-α in AA patients compared to healthy individuals, which was expected due to the nature of AA which seems to be a Th1 dominant autoimmune disease40. Hoffmann et al. showed that TNF-α could abrogate hair growth in vitro; it has been found that TNF-α can result in vacuolation of matrix cells in follicle bulbs and can diminish the matrix size41, 42. Alzolibani et al. showed that mRNA expression of TNF-α is higher in PBMC of AA patients than healthy controls30. It has been found that lesioned skin biopsies of AA patients have elevated levels of TNF-α in comparison with non-lesioned biopsies or biopsies from healthy controls43.
IL-6 is a pro-inflammatory cytokine that plays an important role in the pathogenesis of different autoimmune diseases or chronic inflammatory conditions44. This study showed that IL-6 levels are significantly raised in AA patients compared to healthy individuals. Transcript of IL-6 has been shown to have higher expression in balding dermal papilla cells than non-balding dermal papilla cells and IL-6 can also inhibit elongation of the hair shaft in a dose-dependent manner45. IL-6 levels tend to be elevated in autoimmune diseases regardless of the dominant response; it is elevated in multiple sclerosis patients (Th1 dominant), SLE (TH2 dominant), and psoriasis (in which Th17 plays a crucial role in its pathogenesis). Therefore, elevated levels of IL-6 in AA patients are not unexpected46, 47, 48, 49, 50, 51.
IL-10 is an anti-inflammatory cytokine, and its dysregulation can increase the chance of autoimmunity52. Contrary to our expectations, pooled anti-inflammatory IL-10 was not significantly decreased in patients with AA. The literature on AA proposes that IL-10 imperfection might contribute to its pathogenesis16. It seems that disequilibrium in cytokine production, with a relative surplus of pro-inflammatory and Th1 types versus anti-inflammatory cytokines, could have a role in the permanence of alopecia lesions53. Freyschmidt-Paul et al. found that IL-10 deficient mice are more resistant to the induction of AA54. Hoffman et al. showed increased mRNA levels of IL-10 in AA biopsies after treatment with Diphenylcyclopropenone, and IL-10 transcript levels in untreated biopsies of AA patients were nearly similar to healthy controls55.
Altogether, these lines of evidence show that activation of the immune system is one of the biological processes associated with the pathogenesis of AA. Thus, the present study could be worthwhile in providing an evidence pool for future treatment suggestions.
There were a few limitations associated with our meta-analysis. First, insufficient data in some studies was a significant issue in our conducted meta-analysis. Second, due to the inconsistency of the classification methods, the different disease subtypes of AA could not be separately analyzed using the meta-analysis. Third, the limited number of studies may lead to less accurate results in meta-analysis studies. Fourth, publication bias could affect the results of this meta-analysis study.
Conclusions
Altogether, these lines of evidence show that activation of the immune system is one of the biological processes which may be associated with the pathogenesis of AA. The present study could be worthwhile in providing an evidence pool for future treatment suggestions. Despite of our findings, we still need further researches with a stronger design to develop knowledge in this regard.
Abbreviations
AA: Alopecia areata
IL-6: Interleukin-6
IL-10: Interleukin-10
TNF-α: Tumor Necrosis Factor-α
Acknowledgments
We wish to appreciate all those who helped us during this study. The authors declare that they did not receive any funding for this study.
Author’s contributions
Authors equally contributed to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Wasserman
D.,
Guzman-Sanchez
D.A.,
Scott
K.,
McMichael
A.,
Alopecia areata. Int J Dermatol.
2007;
46
(2)
:
121-31
.
View Article PubMed Google Scholar -
Safavi
K.H.,
Muller
S.A.,
Suman
V.J.,
Moshell
A.N.,
Melton
L.J.,
Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc.
1995;
70
(7)
:
628-33
.
View Article PubMed Google Scholar -
Tan
E.,
Tay
Y.K.,
Goh
C.L.,
Giam
Y. Chin,
The pattern and profile of alopecia areata in Singapore - a study of 219 Asians. Int J Dermatol.
2002;
41
(11)
:
748-53
.
View Article PubMed Google Scholar -
Tan
E.,
Tay
Y.K.,
Giam
Y.C.,
A clinical study of childhood alopecia areata in Singapore. Pediatr Dermatol.
2002;
19
(4)
:
298-301
.
View Article PubMed Google Scholar -
Ting
P.T.,
Barankin
B.,
Patches of hair loss on the scalp. Can Fam Physician.
2006;
52
(8)
:
957
.
PubMed Google Scholar -
Medzhitov
R.,
Origin and physiological roles of inflammation. Nature.
2008;
454
(7203)
:
428-35
.
View Article PubMed Google Scholar -
Wojdasiewicz
P.,
Poniatowski
L.A.,
Szukiewicz
D.,
The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of inflammation.
2014;
2014
:
561459
.
View Article Google Scholar -
Pradhan
A.D.,
Manson
J.E.,
Rifai
N.,
Buring
J.E.,
Ridker
P.M.,
C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA.
2001;
286
(3)
:
327-34
.
View Article PubMed Google Scholar -
Pahwa
R.,
Goyal
A.,
Bansal
P.,
Jialal
I.,
Chronic Inflammation. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2020. 2018
.
-
Malemud
C.J.,
Defective JAK-STAT Pathway Signaling Contributes to Autoimmune Diseases. Curr Pharmacol Rep.
2018;
4
(5)
:
358-66
.
View Article Google Scholar -
Bilgic
O.,
Sivrikaya
A.,
Unlu
A.,
Altinyazar
H.C.,
Serum cytokine and chemokine profiles in patients with alopecia areata. J Dermatolog Treat.
2016;
27
(3)
:
260-3
.
View Article PubMed Google Scholar -
Li
P.,
Zheng
Y.,
Chen
X.,
Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front Pharmacol.
2017;
8
:
460
.
View Article PubMed Google Scholar -
Sokolik
R.,
Iwaszko
M.,
Świerkot
J.,
Wysoczańska
B.,
Korman
L.,
Wiland
P.,
Relationship Between Interleukin-6 -174G/C Genetic Variant and Efficacy of Methotrexate Treatment in Psoriatic Arthritis Patients. Pharm Genomics Pers Med.
2021;
14
:
157-66
.
View Article PubMed Google Scholar -
Ataseven
A.,
Saral
Y.,
Godekmerdan
A.,
Serum cytokine levels and anxiety and depression rates in patients with alopecia areata. Eurasian J Med.
2011;
43
(2)
:
99-102
.
View Article PubMed Google Scholar -
Serarslan
G.,
Özcan
O.,
Okyay
E.,
Ünlü
B.,
Karadağ
M.,
Role of adiponectin and leptin in patients with alopecia areata with scalp hair loss. Ir J Med Sci.
2020;
190
(3)
:
1015-1020
.
View Article PubMed Google Scholar -
Gautam
R.K.,
Singh
Y.,
Gupta
A.,
Arora
P.,
Khurana
A.,
Chitkara
A.,
The profile of cytokines (IL-2, IFN-γ, IL-4, IL-10, IL-17A, and IL-23) in active alopecia areata. J Cosmet Dermatol.
2020;
19
(1)
:
234-40
.
View Article PubMed Google Scholar -
Ma
X.,
Chen
S.,
Jin
W.,
Gao
Y.,
Th1/Th2 PB balance and CD200 expression of patients with active severe alopecia areata. Exp Ther Med.
2017;
13
(6)
:
2883-7
.
View Article PubMed Google Scholar -
Barahmani
N.,
Lopez
A.,
Babu
D.,
Hernandez
M.,
Donley
S.E.,
Duvic
M.,
Serum T helper 1 cytokine levels are greater in patients with alopecia areata regardless of severity or atopy. Clin Exp Dermatol.
2010;
35
(4)
:
409-16
.
View Article PubMed Google Scholar -
Peters
J.P.,
Hooft
L.,
Grolman
W.,
Stegeman
I.,
Reporting Quality of Systematic Reviews and Meta-Analyses of Otorhinolaryngologic Articles Based on the PRISMA Statement. PLoS One.
2015;
10
(8)
:
e0136540
.
View Article PubMed Google Scholar -
National Heart, Lung, and Blood Institute. Study Quality Assessment Tools [https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools]..
.
-
Egger
M.,
Davey Smith
G.,
Schneider
M.,
Minder
C.,
Bias in meta-analysis detected by a simple, graphical test. BMJ.
1997;
315
(7109)
:
629-34
.
View Article PubMed Google Scholar -
Higgins
J.P.,
Green
S.,
Cochrane handbook for systematic reviews of interventionsJohn Wiley & Sons 2011.
Google Scholar -
Atwa
M.A.,
Youssef
N.,
Bayoumy
N.M.,
T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factor-α) in patients with alopecia areata: association with clinical type and severity. Int J Dermatol.
2016;
55
(6)
:
666-72
.
View Article PubMed Google Scholar -
Tomaszewska
K.,
Koz\lowska
M.,
Kaszuba
A.,
Lesiak
A.,
Narbutt
J.,
Zalewska-Janowska
A.,
Increased Serum Levels of IFN-γ, IL-1β, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid Med Cell Longev.
2020;
2020
:
5693572
.
View Article PubMed Google Scholar -
Loh
S.H.,
Moon
H.N.,
Lew
B.L.,
Sim
W.Y.,
Role of T helper 17 cells and T regulatory cells in alopecia areata: comparison of lesion and serum cytokine between controls and patients. J Eur Acad Dermatol Venereol.
2018;
32
(6)
:
1028-33
.
View Article PubMed Google Scholar -
Tembhre
M.K.,
Sharma
V.K.,
T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br J Dermatol.
2013;
169
(3)
:
543-8
.
View Article PubMed Google Scholar -
Abd El-Raheem
T.,
Mahmoud
R.H.,
Hefzy
E.M.,
Masoud
M.,
Ismail
R.,
Aboraia
N.M.,
Tumor necrosis factor (TNF)-α- 308 G/A gene polymorphism (rs1800629) in Egyptian patients with alopecia areata and vitiligo, a laboratory and in silico analysis. PLoS One.
2020;
15
(12)
:
e0240221
.
View Article PubMed Google Scholar -
Abdel Halim
D.,
Abu Zeid
O.M.,
Rashed
L.,
Saleh
M.A.,
Alteration of serum and tissue tumor necrosis factor alpha levels: A possible mechanism of action of oral pulse steroids in the treatment of alopecia areata. J Cosmet Dermatol.
2019;
18
(4)
:
1128-32
.
View Article PubMed Google Scholar -
Kasumagic-Halilovic
E.,
Prohic
A.,
Cavaljuga
S.,
Tumor necrosis factor-alpha in patients with alopecia areata. Indian J Dermatol.
2011;
56
(5)
:
494-6
.
View Article PubMed Google Scholar -
Omar
S.I.,
Hamza
A.M.,
Eldabah
N.,
Habiba
D.A.,
IFN-α and TNF-α serum levels and their association with disease severity in Egyptian children and adults with alopecia areata. Int J Dermatol.
2021;
60
(11)
:
1397-1404
.
View Article PubMed Google Scholar -
Teraki
Y.,
Imanishi
K.,
Shiohara
T.,
Cytokines in alopecia areata: contrasting cytokine profiles in localized form and extensive form (alopecia universalis). Acta Derm Venereol.
1996;
76
(6)
:
421-3
.
PubMed Google Scholar -
Alzolibani
A.A.,
Rasheed
Z.,
Bin Saif
G.,
Al-Dhubaibi
M.S.,
Al Robaee
A.A.,
Altered expression of intracellular Toll-like receptors in peripheral blood mononuclear cells from patients with alopecia areata. BBA Clin.
2016;
5
:
134-42
.
View Article PubMed Google Scholar -
Simakou
T.,
Butcher
J.P.,
Reid
S.,
Henriquez
F.L.,
Alopecia areata: A multifactorial autoimmune condition. J Autoimmun.
2019;
98
:
74-85
.
View Article PubMed Google Scholar -
Pratt
C.H.,
King
L.E.,
Messenger
A.G.,
Christiano
A.M.,
Sundberg
J.P.,
Alopecia areata. Nat Rev Dis Primers.
2017;
3
(1)
:
17011
.
View Article PubMed Google Scholar -
Gregoriou
S.,
Papafragkaki
D.,
Kontochristopoulos
G.,
Rallis
E.,
Kalogeromitros
D.,
Rigopoulos
D.,
Cytokines and other mediators in alopecia areata. Mediators Inflamm.
2010;
2010
:
928030
.
View Article PubMed Google Scholar -
Tabara
K.,
Kozłowska
M.,
Jkedrowiak
A.,
Bienias
W.,
Kaszuba
A.,
Serum concentrations of selected proinflammatory cytokines in children with alopecia areata. Postepy Dermatol Alergol.
2019;
36
(1)
:
63-69
.
View Article PubMed Google Scholar -
Zhang
B.,
Ramesh
G.,
Norbury
C.C.,
Reeves
W.B.,
Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int.
2007;
72
(1)
:
37-44
.
View Article PubMed Google Scholar -
Moelants
E.A.,
Mortier
A.,
Van Damme
J.,
Proost
P.,
Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol Cell Biol.
2013;
91
(6)
:
393-401
.
View Article PubMed Google Scholar -
Ogawa
E.,
Sato
Y.,
Minagawa
A.,
Okuyama
R.,
Pathogenesis of psoriasis and development of treatment. J Dermatol.
2018;
45
(3)
:
264-72
.
View Article PubMed Google Scholar -
Gilhar
A.,
Kalish
R.S.,
Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev.
2006;
5
(1)
:
64-9
.
View Article PubMed Google Scholar -
Hoffmann
R.,
Eicheler
W.,
Huth
A.,
Wenzel
E.,
Happle
R.,
Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res.
1996;
288
(3)
:
153-6
.
View Article PubMed Google Scholar -
Philpott
M.P.,
Sanders
D.A.,
Bowen
J.,
Kealey
T.,
Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-α in alopecia areata. Br J Dermatol.
1996;
135
(6)
:
942-8
.
View Article PubMed Google Scholar -
El-Tahlawi
S.R.,
El-Hanafy
G.M.,
El-Rifaie
A.E.,
Shaker
O.G.,
Assessment of the level of tumor necrosis factor-α in localized alopecia areata. Journal of the Egyptian Women's Dermatologic Society..
2013;
10
(2)
:
81-4
.
View Article Google Scholar -
Yang
D.-H.,
The Biological Effects of Interleukin-6 and Their Clinical Applications in Autoimmune Diseases and Cancers. Rheumatica Acta: Open Access.
2017;
1
(1)
:
6-16
.
-
Kwack
M.H.,
Ahn
J.S.,
Kim
M.K.,
Kim
J.C.,
Sung
Y.K.,
Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol.
2012;
132
(1)
:
43-9
.
View Article PubMed Google Scholar -
Stelmasiak
Z.,
Kozio\l-Montewka
M.,
Dobosz
B.,
Rejdak
K.,
Bartosik-Psujek
H.,
Mitosek-Szewczyk
K.,
Interleukin-6 concentration in serum and cerebrospinal fluid in multiple sclerosis patients. Med Sci Monit.
2000;
6
(6)
:
1104-8
.
PubMed Google Scholar -
Ripley
B.J.,
Goncalves
B.,
Isenberg
D.A.,
Latchman
D.S.,
Rahman
A.,
Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis.
2005;
64
(6)
:
849-53
.
View Article PubMed Google Scholar -
Ishida
H.,
Ota
H.,
Yanagida
H.,
Dobashi
H.,
An imbalance between Th1 and Th2-like cytokines in patients with autoimmune diseases--differential diagnosis between Th1 dominant autoimmune diseases and Th2 dominant autoimmune diseases. Jpn J Clin Med.
1997;
55
(6)
:
1438-43
.
PubMed Google Scholar -
Li
B.,
Huang
L.,
Lv
P.,
Li
X.,
Liu
G.,
Chen
Y.,
The role of Th17 cells in psoriasis. Immunol Res.
2020;
68
(5)
:
296-309
.
View Article PubMed Google Scholar -
Fitch
E.,
Harper
E.,
Skorcheva
I.,
Kurtz
S.E.,
Blauvelt
A.,
Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep.
2007;
9
(6)
:
461-7
.
View Article PubMed Google Scholar -
P.S. Oliveira,
P.R. Cardoso,
E.V. Lima,
M.C. Pereira,
A.L. Duarte,
I.D. Pitta,
M.J. Rêgo,
M.G. Pitta,
IL-17A, IL-22, IL-6, and IL-21 Serum Levels in Plaque-Type Psoriasis in Brazilian Patients. Mediators Inflamm.
2015;
2015
:
819149
.
View Article PubMed Google Scholar -
Iyer
S.S.,
Cheng
G.,
Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol.
2012;
32
(1)
:
23-63
.
View Article Google Scholar -
Bodemer
C.,
Peuchmaur
M.,
Fraitaig
S.,
Chatenoud
L.,
Brousse
N.,
De Prost
Y.,
Role of cytotoxic T cells in chronic alopecia areata. J Invest Dermatol.
2000;
114
(1)
:
112-6
.
View Article PubMed Google Scholar -
Freyschmidt-Paul
P.,
McElwee
K.J.,
Happle
R.,
Kissling
S.,
Wenzel
E.,
Sundberg
J.P.,
Interleukin-10-deficient mice are less susceptible to the induction of alopecia areata. J Invest Dermatol.
2002;
119
(4)
:
980-2
.
View Article PubMed Google Scholar -
Hoffmann
R.,
Wenzel
E.,
Huth
A.,
van der Steen
P.,
Schäufele
M.,
Henninger
H.P.,
Cytokine mRNA levels in Alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. J Invest Dermatol.
1994;
103
(4)
:
530-3
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 10 (2021)
Page No.: 4668-4678
Published on: 2021-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4337 times
- PDF downloaded - 978 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress