Abstract
Background & objectives: Programmed Death Ligand 1 (PD-L1) is a putative biomarker response to an immune checkpoint blockade that is related to poor outcomes as well as treatment strategy of numerous carcinomas, including gastric cancer. However, there is still a lack of other biomarkers that can predict patient prognosis in clinical settings. For this reason, we investigated PD-L1 expression and Combined Positive Score (CPS), as well its association with histopathological characteristics in gastric cancer patients.
Methods: We collected clinicopathological data from 54 patients with gastric cancer who underwent surgery at 103 Military Medical Hospital, Vietnam, from December 2018 to November 2020. Immunohistochemistry staining of the tumor specimens for PD-L1 expression and CPS were evaluated and accessed relationship to patient characteristics and overall survival.
Results: PD-L1 with strong and moderate expression was 1.9% and 20.3%, and CPS >= 1 was 46.3%. PD-L1 expression and CPS had no statistical relationship with histopathological characteristics, with the exception that tumor location, which was significantly related to PD-L1 expression (p = 0.001). PD-L1 positivity and CPS1 were related to worse overall survival of gastric cancer patients.
Conclusions: Our data indicated that PD-L1 and CPS are independent prognostic markers and indicators for use in targeted therapy for gastric cancer patients.
Introduction
Gastric cancer (GC) is the fifth most common cancer globally1. Most cases have been reported in Eastern Asia, particularly in China, Japan, and South Korea2. According to the 2020 GLOBOCAN, gastric cancer was ranked as the fourth most common cancer in Vietnam after liver, lung, and breast cancers, with an average number of new cases of 17,906, with the 5-year prevalence rate at 24.64 per 100,0003. The causes of gastric cancer are still unclear, but some factors have been shown to increase risk. The most common risk factor is the microbe Helicobacter pylori, which contributes to cancer development4. Diet and lifestyle risk factors include obesity, a diet high in sodium and low in vegetables, smoked foods, smoking, and alcohol consumption. The cancer can also develop from some stomach diseases, such as gastroesophageal reflux, chronic gastritis, and stomach polyps. Hereditary factors also increase risk of contracting gastric cancer5.
Cancer immunotherapy has shown promising results in recent years. In some types of cancer, interaction between programmed death-ligand 1 (PD-L1) in cancer cells and programmed death-1 (PD-1) in T cells inhibits T cell function, thus helping to prevent the cancer cells from evading the immune system6. Currently, there are several approaches to locking interaction between PD-L1 and PD-1, such as the use of gene therapy or antagonistic monoclonal antibodies. Previously, a study by Su et al. used gene therapy to reprogram T cells by eliminating the PD-1 gene using the CRISPR-Cas9 technique; the results showed a significant reduction of PD-1 expression but did not affect the viability of T cells, suggesting a new strategy for cancer treatment7. Alternatively, the use of monoclonal antibodies to block immune checkpoint molecules has been shown to be a promising therapeutic strategy against several types of cancer8, 9 and the immune regulatory PD-1/PD-L1 axis has been used for immunotherapy for gastric cancer10. The National Comprehensive Cancer Network (NCCN) guidelines suggest that immunotherapeutic strategies be applied to patients with advanced-stage GC11. Some clinical trials have indicated that monoclonal antibodies targeting PD-L1 or its receptor PD-1 inhibit the PD-1/PD-L1 signaling pathway and enhance T-cell function, which improves outcomes in patients with GC, particularly in the advanced stage12, 13, 14. In a trial from CheckMate 032 (GC/GEJC cohort), there was a strong association between the combined assessment of PD-L1 expression with the CPS and the effectiveness of anti-PD-1 therapy15.
Beyond assessment PD-L1 expression and CPS, there are currently no biomarkers for evaluating the clinical efficacy of anti-PD-1/PD-L1 immunotherapy. In Vietnam, the evaluation of PD-L1 expression and CPS in gastric cancer patients is limited, thus constraining the access to anti-PD-1/PD-L1 therapy. Additionally, determining the association between PD-L1 expression and pathological features is essential but still controversial and not well-elucidated. Therefore, we investigate the relationship between PD-L1 expression with clinicopathological characteristics and the survival of Vietnamese patients with gastric cancer.
Methods
Patient cohort
We retrospectively enrolled 54 GC patients who had undergone curative gastrectomy at 103 Military Medical Hospital, Vietnam, between December 2018 and November 2020. All patients were diagnosed using pathological results of H&E staining specimens to identify gastric cancer and also underwent gastrectomy and scraping of regional nodes. These patients were not treated with chemoradiotherapy beforehand and were monitored after surgery. We selected patients with recurrent GC, metastatic cancer to the stomach, or a combination of these and other cancers. The Ethics Committee of 103 Military Medical Hospital approved all study procedures (code: 140/2016/IRB-MH103).
Patient characteristics
We collected data on patient features including tumor location, tumor size, gross appearance (based on the Bormann classification), histologic type (based on the Lauren classification), histopathology classification (based on the WHO classification of Tumors of the Digestive System, 201016), tumor differentiation, depth of invasion (T stage according to the 8th edition of the UICC/AJCC TNM classification17), lymph node status, and vascular invasion.
PD-L1 Immunohistochemistry
Tumor specimens in a paraffin-embedded block were cut into 3 µm thick sections. We carried out immunohistochemical (IHC) staining for PD-L1 (clone 73-10, rabbit anti-human monoclonal antibody, 10 mg/ml, Leica, UK) on an automated slide stainer (Leica ST5010 Auto Stainer XL, Leica Biosystems), following manufacturer instructions. The samples were deparaffinized in xylene and rehydrated using a series of ethanol. Sections were incubated with the primary antibody for 30 minutes, then the secondary antibody of biotin-labeled anti-rabbit IgG was applied for 8 minutes. After washing in PBS, signals were visualized by incubation with polymer solution for 8 minutes and then 3-3’-diaminobenzidine (DAB) for 30 seconds. Slides were counterstained with hematoxylin for 6 minutes before mounting. After each step, wash through Bond-was solution two times in 2 minutes. Positive and negative controls were used for IHC reactions. Tonsils were used as the positive control, as PD-L1 should show strong staining in the crypt epithelium and weak to moderate staining of the follicular macrophages in the germinal centers.
Evaluation of PD-L1 expression and CPS scoring
We evaluated the expression of PD-L1 and calculated CPS following the protocol of PD-L1 IHC 22C3 pharmDx, which is FDA-approved for in vitro diagnostic use18. We randomly examined four fields on the tumor site slide of each specimen at 400X magnification. The expression of PD-L1 was recorded according to the percentage of tumor-stained cells per total viable tumor cells. We defined less than 1%, 1 — 49%, and ≥ 50% as weak, moderate, and strong expressions, respectively. For calculating CPS, we used the formula as follows:
Based on CPS, we divided samples into two groups: CPS < 1 and CPS ≥ 1.
Statistical analysis
After collecting sufficient information, the data for categorical variables was shown by frequency and percentage. We used a chi-square test to analyze correlations between PD-L1 expression, CPS, and clinicopathological features. We used the Kaplan-Meier method to construct the survival curves and a log-rank test for survival comparison. Statistical analysis was performed using SPSS software ver. 20.0 (IBM, Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
| Characteristics | (%) | |
|---|---|---|
| Tumor location | Body | 3 (5.5) |
| Lesser curvature | 17 (31.5) | |
| Pylorus | 34 (63) | |
| Tumor size | < 5cm | 43 (79.6) |
| ≥ 5cm | 11 (20.4) | |
| Bormann classification | Polyp | 3 (5.6) |
| Fungating | 6 (11.1) | |
| Ulcerous-infiltrative | 37 (68.5) | |
| Diffuse-infiltrative | 8 (14.8) | |
| Lauren classification | Diffuse | 33 (61.1) |
| Intestinal | 8 (14.8) | |
| Mixed | 13 (24.1) | |
| WHO classification | Tubular adenocarcinoma | 40 (74.1) |
| Mucinous adenocarcinoma | 3 (5.6) | |
| Signet ring cell carcinoma | 3 (5.6) | |
| Undifferentiated carcinoma | 8 (14.8) | |
| Tumor differentiation | High | 5 (9.3) |
| Mediated | 22 (40.7) | |
| Poor | 17 (31.5) | |
| Undifferentiated | 10 (18.5) | |
| Depth of invasion (T stage) | T1 | 2 (3.7) |
| T2 | 11 (20.4) | |
| T3 | 22 (40.7) | |
| T4 | 19 (35.2) | |
| Lymph node metastasis | Positive | 27 (50) |
| Negative | 27 (50) | |
| Vascular invasion | Positive | 3 (5.6) |
| Negative | 51 (94.4) |
| Parameters | (%) | |
|---|---|---|
| PD-L1 expression | Weak | 42 (77.8) |
| Moderate | 11 (20.3) | |
| Strong | 1 (1.9) | |
| CPS | < 1 | 29 (53.7) |
| ≥ 1 | 25 (46.3) |
| Characteristics | PD-L1, n (%) | p | CPS, n (%) | p | |||
|---|---|---|---|---|---|---|---|
| Weak | Moderate | Strong | < 1 | ≥ 1 | |||
| Tumour location | |||||||
| Cardia | 2 (66.7) | 0 (0.0) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 0.442 | |
| Lesser curvature | 13 (76.5) | 4 (23.5) | 0 (0.0) | 0.001 | 7 (41.2) | 10 (58.8) | |
| Polyrus | 27 (79.4) | 7 (20.6) | 0 (0.0) | 20 (58.8) | 14 (41.2) | ||
| Tumour size | |||||||
| < 5cm | 33 (76.7) | 9 (20.9) | 1 (2.3) | 0.853 | 23 (53.5) | 20 (46.5) | 0.950 |
| ≥ 5cm | 9 (81.8) | 2 (18.2) | 0 (0.0) | 6 (54.5) | 5 (45.5) | ||
| Bormann classification | |||||||
| Polyp type | 2 (66.7) | 1 (33.3) | 0 (0.0) | 0.473 | 2 (66.7) | 1 (33.3) | 0.178 |
| Fungating | 6 (100.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 4 (66.7) | ||
| Ulcerous-infiltrative | 26 (70.3) | 10 (27.0) | 1 (2.7) | 23 (62.2) | 14 (37.8) | ||
| Diffuse-infultrative | 8 (100.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) | 6 (75.0) | ||
| Lauren classification | |||||||
| Diffuse | 26 (78.8) | 6 (18.2) | 1 (3.0) | 0.759 | 18 (54.5) | 15 (45.5) | 0.950 |
| Intestinal | 7 (87.5) | 1 (12.5) | 0 (0.0) | 4 (50.0) | 4 (50.0) | ||
| Mixed | 9 (69.2) | 4 (30.8) | 0 (0.0) | 6 (50.0) | 6 (50.0) | ||
| WHO classification | |||||||
| Tubular adenocarcinoma | 30 (75.0) | 9 (22.5) | 1 (2.5) | 0.903 | 20 (50.0) | 20 (50.0) | 0.383 |
| Mucinous denocarcinoma | 3 (100.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | ||
| Signet ring cell carcinoma | 3 (100.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 1 (33.3) | ||
| Undifferentiated arcinoma | 6 (75.0) | 2 (25.0) | 0 (0.0) | 4 (50.0) | 4 (50.0) | ||
| Tumour differentiation | |||||||
| High | 5 (100.0) | 0 (0.0) | 0 (0.0) | 0.586 | 3 (60.0) | 2 (40.0) | 0.986 |
| Mediated | 18 (81.8) | 4 (18.2) | 0 (0.0) | 12 (54.5) | 10 (45.5) | ||
| Poor | 11 (64.7) | 5 (29.4) | 1 (5.9) | 9 (52.9) | 8 (47.1) | ||
| Undifferentiated | 8 (80.0) | 2 (20.0) | 0 (0.0) | 5 (50.0) | 5 (50.0) | ||
| Depth of invasion (T stage) | |||||||
| T1 | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0.873 | 1 (50.0) | 1 (50.0) | 0.902 |
| T2 | 9 (81.8) | 2 (18.2) | 0 (0.0) | 6 (54.5) | 5 (45.5) | ||
| T3 | 17 (77.3) | 4 (18.2) | 1 (4.5) | 13 (59.1) | 9 (40.9) | ||
| T4 | 14 (73.7) | 5 (26.3) | 0 (0.0) | 9 (47.4) | 10 (52.6) | ||
| Lymph node metastasis | |||||||
| Positive | 22 (81.5) | 4 (14.8) | 1 (3.7) | 0.384 | 13 (48.1) | 14 (59.1) | 0.413 |
| Negative | 20 (74.1) | 7 (25.9) | 0 (0.0) | 16 (59.3) | 11 (40.7) | ||
| Vascular invasion | |||||||
| Positive | 1 (33.3) | 2 (66.7) | 0 (0.0) | 0.122 | 1 (33.3) | 2 (66.7) | 0.467 |
| Negative | 41 (80.4) | 9 (17.6) | 1 (2.0) | 28 (54.9) | 23 (45.1) |
Results
Patients' clinicopathologic data and relationship with PD-L1 expression
We summarize the pathological characteristics of patients in Table 1. Results demonstrated that tumor location was the most common site in pylorus with 63% prevalence, followed by curvature at 31.5% and 5% in the body. Tumor size < 5 cm accounted for 79.6% of patients, whereas tumor size of ≥ 5 cm accounted for 20.4%. Borman classification was ulcerous-infiltrative for 68.5% of patients, diffuse-infiltrative for 14.8%, fungating for 11.1%, and polypoid for 5.6%. Lauren classification was diffuse for 61%, intestinal for 14.8%, and mixed for 24.1%. The WHO classification was tubular adenocarcinoma for 74.1%, mucinous adenocarcinoma, signet-ring cell carcinoma for 5.6%, and undifferentiated carcinoma for 14.8%. The tumor differentiation was high at 9.3%, moderate at 40.7%, low at 31.5%, and undifferentiated at 18.5%. Depth of invasion was T1 at 3.7%, T2 at 20.4%, T3 at 40.7%, and T4 at 35.2%. Lymph node metastasis was 50% for both positive and negative. Vascular invasion was 5.6% for positive and 94.4% for negative.
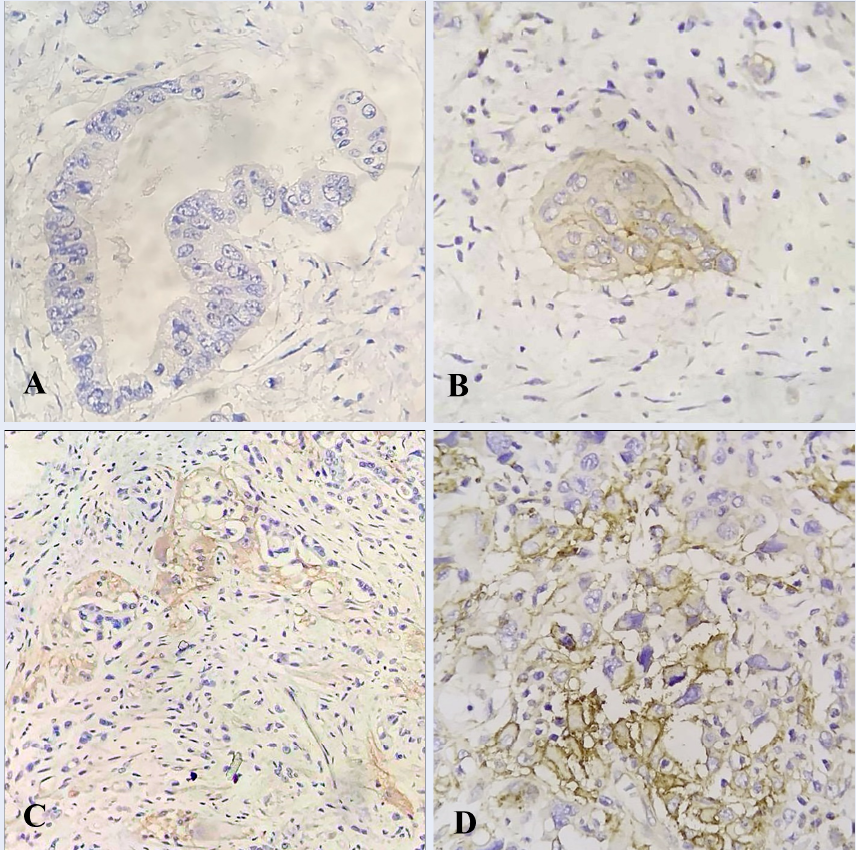
IHC results for PD-L1 expression are shown in Figure 1 and summarized in Table 2 with rates of weak expression at 77.8%, moderate expression at 20.3%, and strong expression at 1.9%. The corresponding rankings of CPS < 1 accounted for 53.7%, while CPS ≥ 1 accounted for 46.3% of patients.
We summarize the relationship between PD-L1 expression and CPS and pathological characteristics of GC patients in Table 3. The results show no statistically significant relationship between PD-L1 expression and CPS and tumor size, Bormann classification, Lauren classification, WHO classification, tumor differentiation, depth of invasion, lymph node metastasis, or vascular invasion. Tumor location was the exception, being significantly related to PD-L1 expression (p = 0.001, chi-square test).
Impact of PD-L1 expression and CPS on the survival of patients with gastric cancer
We evaluated PD-L1 expression and CPS with regard to overall survival in gastric cancer patients. This analysis defined samples with PD-L1 strong and moderate expression as positive and samples with weak expression as negative. Based on PD-L1 expression and CPS, overall survival is represented by the Kaplan–Meier curves in Figure 2. PD-L1-positive patients had lower overall survival than PD-L1-negative patients; the difference was statistically significant, with p = 0.001 (Figure 2 A). Patients with CPS ≥ 1 had lower overall survival than patients with CPS < 1; the difference was statistically significant, with p = 0.047 (Figure 2 B). The results showed a relationship between the expression of PD-L1 and CPS and the prognosis of gastric cancer patients.
Discussion
In this study, we divided PD-L1 expression into weak, moderate, and strong rankings, with ratios of 77.8%, 20.3%, and 1.9%, respectively. For comparison with other studies, we defined samples with PD-L1 strong and moderate expression as positive, with a combined percentage of 22.2% presence in patients (Table 2). Our results are similar results found in studies by Tatsuro Tamura et al. (29.6%)14, Kawazoe et al. (24.8%)19, and Boger et al. (23.73%)20. Our figure is, however, higher than that shown in some studies, such as Kang et al. (15.38%)21 and Dai et al. (14.32%)22. Conversely, PD-L1 positivity was shown to be much higher in some other studies, such as those by Lin Zhang et al. (50.8%)23 or KEYNOTE-059 (57.1%)24. Despite many studies on PD-L1 expression, only a few authors accounted for both PD-L1 positivity and CPS in patients with GC. Some recent reports have suggested immunotherapy for patients with advanced gastric cancer and CPS ≥ 125, 26. CPS measures PD-L1 staining in both tumor and immune cells. In immune cells, PD-L1 expression is essential for inducing immune tolerance27. Therefore, instead of only assessing PD-L1 positivity in tumor cells, CPS calculation also measures the colorization of immune function cells, such as lymphocytes and macrophages. Thus, the CPS ≥ 1 ratio was higher than the PD-L1 positivity ratio in our study. Patients with CPS ≥ 1 in our study accounted for 46.3% of participants (Table 2), which is lower than the results found by Shitara et al. (67%)26 or Yamashita et al. (71.7%)28. The percentages of PD-L1 positivity and CPS ≥ 1 in our study differ from other studies and may depend on factors like patient cohort, genotype differences, histopathological characteristics, immunohistochemical staining methods used, or cut-off values for distinguishing between positive and negative PD-L1 expression.
Our results showed no relationship between the expression levels of PD-L1, CPS, and histopathological characteristics in gastric cancer patients, except regarding tumor location, which was significantly related to the expression level of PD-L1 (p = 0.001, chi-square test, Table 3). Results from other studies suggest that PD-L1 overexpression is associated with depth of invasion (T stage)20, lymph node metastasis29, 30, and vascular invasion31. In contrast, there has not been any demonstrated association of PD-L1 expression with tumor location32, tumor size33, tumor differentiation34, Lauren classification22, or invasion the lymphatic system21. This suggests that the relationship between PD-L1 expression and pathological characteristics varies widely between studies.This study also showed that PD-L1 with weak expression had significantly more prolonged survival than PD-L1 with moderate and strong expression (p = 0.001, Figure 2 A). Other studies have shown similar results; patients with PD-L1 overexpression have been shown to have a shorter survival time and a worse prognosis than patients with weak or negative PD-L1 expression35, 36, 37. Our results show that patients with CPS < 1 had significantly greater prolonged survival than patients with CPS ≥ 1 (p = 0.047, Figure 2 B). This was similar to the results from Yamashita’s study28, which also showed that these patients had a significantly shorter overall survival than those with CPS < 1 (p = 0.017). CPS has better prognostic significance than TPS (Tumor Positive Score), suggesting that, in addition to PD-L1-stained cells, the presence of lymphocytes surrounding the tumors and in the stroma have a significant effect on the overall survival of the patient. Therefore, CPS is an essential indicator for immunotherapy. Schoemig-Markiefka et al. reported that the association between expression of PD-L1 and CPS was related to the assessment of responsiveness to PD-L1 inhibitor target therapy, in which CPS ≥ 1 increases the response to PD-L1 inhibitor38. The results of our study show that the combination of assessment of PD-L1 expression and CPS can be a factor in helping predict and consider immunotherapy in gastric cancer patients. We believe these findings will help us improve therapeutic strategies in future clinical trials for treatment with PD-1/PD-L1 inhibitors.
This study also had some limitations. The sample number was small, resulting in an analytical evaluation was not comprehensive. Additionally, the cut-off values for distinguishing between positive and negative PD-L1 expression still hold room for improvement.
Conclusions
Overexpression of PD-L1 and CPS ≥ 1 contribute to poor prognosis and reduced survival in gastric cancer patients. Our study indicates that CPS and PD-L1 expression are independent prognostic indicators, and that combination of these two indicators can improve strategies for targeting PD-1/PD-L1 inhibitors in therapy for gastric cancer patients.
Abbreviations
AJCC: American Joint Committee on Cancer; CPS: Combined positive score; DAB: 3-3’-diaminobenzidine; FDA: Food and Drug Administration; GC: Gastric cancer; GC/GEJC: Gastric or gastroesophageal junction cancer; GLOBOCAN: Global Cancer Incidence, Mortality And Prevalence; IBM: International Business Machines Corporation; IHC: Immunohistochemical; NCCN: National Comprehensive Cancer Network; NY: New York; PBS: Phosphate buffered saline; PD-1: Programmed death 1; PD-L1: Programmed Death Ligand 1; SPSS: Statistical Package for the Social Sciences; TNM: Tumor-node-metastasis; UICC: Union for International Cancer Control; UK: United Kingdom; USA: United States of America
Acknowledgments
We would like to thank the patients for joining the study and gratefully acknowledge the support for equipment and techniques from the Leica Biosystems branch in Vietnam.
Author’s contributions
Conceived and designed the study: TND NMH. Patient characteristics data and sample collection: TND NMH DTT TDT NTL NKT PVT NBD NMH DTC. Analyzed and interpreted the data: TND NMH TDT NKT DTC. Contributed to drafting and writing the article: TND NMH DTT TDT DTC. Involved in all aspects of the work through correspondence: NMH DTC. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All patients provided informed consent for participation in the study. The Ethics Committee of 103 Military Medical Hospital (code: 140/2016/IRB-MH103) approved all study procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Sung
H.,
Ferlay
J.,
Siegel
R.L.,
Laversanne
M.,
Soerjomataram
I.,
Jemal
A.,
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal for Clinicians.
2021;
71
(3)
:
209-49
.
View Article PubMed Google Scholar -
Gu
L.,
Chen
M.,
Guo
D.,
Zhu
H.,
Zhang
W.,
Pan
J.,
PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One.
2017;
12
(8)
:
e0182692
.
View Article PubMed Google Scholar -
Cancer IAfRo. Viet Nam Source: Globocan 2020 2021 [Available from: https://gco.iarc.fr/today/home..
.
-
Yahya
E.A.,
Allaq
A.,
Recent Advances in the Role of Microorganisms in Cancer Incidence: Mechanisms and Health Precautions. Biomedical Research and Therapy.
2021;
8
(9)
:
4525-39
.
View Article Google Scholar -
Mayoclinic. Stomach cancer - Symptoms and causes: Mayoclinic; 2022 [Available from: https://www.mayoclinic.org/diseases-conditions/stomach-cancer/symptoms-causes/syc-20352438.. 2022
.
-
Syn
N.L.,
Teng
M.W.,
Mok
T.S.,
Soo
R.A.,
De-novo and acquired resistance to immune checkpoint targeting. The Lancet. Oncology.
2017;
18
(12)
:
e731-41
.
View Article PubMed Google Scholar -
Su
S.,
Hu
B.,
Shao
J.,
Shen
B.,
Du
J.,
Du
Y.,
CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients. Scientific Reports.
2016;
6
(1)
:
20070
.
View Article PubMed Google Scholar -
Robert
C.,
Long
G.V.,
Brady
B.,
Dutriaux
C.,
Maio
M.,
Mortier
L.,
Nivolumab in previously untreated melanoma without BRAF mutation. The New England Journal of Medicine.
2015;
372
(4)
:
320-30
.
View Article PubMed Google Scholar -
Fuchs
C.S.,
Doi
T.,
Jang
R.W.,
Muro
K.,
Satoh
T.,
Machado
M.,
Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncology.
2018;
4
(5)
:
e180013-e
.
View Article PubMed Google Scholar -
Chen
L.T.,
Satoh
T.,
Ryu
M.H.,
Chao
Y.,
Kato
K.,
Chung
H.C.,
A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer.
2020;
23
(3)
:
510-9
.
View Article PubMed Google Scholar -
Network NCC. NCCN Guidelines Gastric Cancer [Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434..
.
-
Keir
M.E.,
Butte
M.J.,
Freeman
G.J.,
Sharpe
A.H.,
PD-1 and its ligands in tolerance and immunity. Annual Review of Immunology.
2008;
26
(1)
:
677-704
.
View Article PubMed Google Scholar -
Svensson
M.C.,
Borg
D.,
Zhang
C.,
Hedner
C.,
Nodin
B.,
Uhlén
M.,
Expression of PD-L1 and PD-1 in Chemoradiotherapy-Naïve Esophageal and Gastric Adenocarcinoma: Relationship With Mismatch Repair Status and Survival. Frontiers in Oncology.
2019;
9
:
136
.
View Article PubMed Google Scholar -
Tamura
T.,
Ohira
M.,
Tanaka
H.,
Muguruma
K.,
Toyokawa
T.,
Kubo
N.,
Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancer. Anticancer Research.
2015;
35
(10)
:
5369-76
.
PubMed Google Scholar -
Lei
M.,
Siemers
N.O.,
Pandya
D.,
Chang
H.,
Sanchez
T.,
Harbison
C.,
Analyses of PD-L1 and Inflammatory Gene Expression Association with Efficacy of Nivolumab ± Ipilimumab in Gastric Cancer/Gastroesophageal Junction Cancer. Clinical Cancer Research.
2021;
27
(14)
:
3926-35
.
View Article PubMed Google Scholar -
Li
Z.S.,
Li
Q.,
[The latest 2010 WHO classification of tumors of digestive system]. Zhonghua bing li xue za zhi = Chinese journal of pathology.
2011;
40
(5)
:
351-354
.
PubMed Google Scholar -
Brierley
J.D.,
Gospodarowicz
M.K.,
Wittekind
C.,
TNM classification of malignant tumours: John Wiley & Sons; 2017 .
Google Scholar -
Solutions
D.A.P.,
PD‐L1 IHC 22C3 pharmDx is CE‐IVD‐marked for in vitro diagnostic use.
.
-
Kawazoe
A.,
Kuwata
T.,
Kuboki
Y.,
Shitara
K.,
Nagatsuma
A.K.,
Aizawa
M.,
Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer.
2017;
20
(3)
:
407-15
.
View Article PubMed Google Scholar -
Böger
C.,
Behrens
H.M.,
Mathiak
M.,
Krüger
S.,
Kalthoff
H.,
Röcken
C.,
PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget.
2016;
7
(17)
:
24269-83
.
View Article PubMed Google Scholar -
Kang
H.J.,
Lee
I.S.,
Park
Y.S.,
Ho
W.J.,
Sohn
D.,
Ahn
J.Y.,
Biomarkers of ebv-positive gastric cancers: loss of pten expression is associated with poor prognosis and nodal metastasis. Annals of Surgical Oncology.
2016;
23
(11)
:
3684-92
.
View Article PubMed Google Scholar -
Dai
C.,
Geng
R.,
Wang
C.,
Wong
A.,
Qing
M.,
Hu
J.,
Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Molecular Oncology.
2016;
10
(10)
:
1551-8
.
View Article PubMed Google Scholar -
Zhang
L.,
Qiu
M.,
Jin
Y.,
Ji
J.,
Li
B.,
Wang
X.,
Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. International Journal of Clinical and Experimental Pathology.
2015;
8
(9)
:
11084-91
.
PubMed Google Scholar -
Fuchs
C.S.,
Doi
T.,
Jang
R.W.,
Muro
K.,
Satoh
T.,
Machado
M.,
Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA oncology.
2018;
4
(5)
:
e180013-e
.
View Article PubMed Google Scholar -
Kang
Y.K.,
Boku
N.,
Satoh
T.,
Ryu
M.H.,
Chao
Y.,
Kato
K.,
Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet.
2017;
390
(10111)
:
2461-71
.
View Article PubMed Google Scholar -
Shitara
K.,
Özgüroğlu
M.,
Bang
Y.J.,
Di Bartolomeo
M.,
Mandalà
M.,
Ryu
M.H.,
investigators
KEYNOTE-061,
Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet.
2018;
392
(10142)
:
123-33
.
View Article PubMed Google Scholar -
Havel
J.J.,
Chowell
D.,
Chan
T.A.,
The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews. Cancer.
2019;
19
(3)
:
133-50
.
View Article PubMed Google Scholar -
Yamashita
K.,
Iwatsuki
M.,
Harada
K.,
Eto
K.,
Hiyoshi
Y.,
Ishimoto
T.,
Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer.
2020;
23
(1)
:
95-104
.
View Article PubMed Google Scholar -
Chang
H.,
Jung
W.Y.,
Kang
Y.,
Lee
H.,
Kim
A.,
Kim
H.K.,
Programmed death-ligand 1 expression in gastric adenocarcinoma is a poor prognostic factor in a high CD8+ tumor infiltrating lymphocytes group. Oncotarget.
2016;
7
(49)
:
80426-34
.
View Article PubMed Google Scholar -
Geng
Y.,
Wang
H.,
Lu
C.,
Li
Q.,
Xu
B.,
Jiang
J.,
Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. International Journal of Clinical Oncology.
2015;
20
(2)
:
273-81
.
View Article PubMed Google Scholar -
Tamura
T.,
Ohira
M.,
Tanaka
H.,
Muguruma
K.,
Toyokawa
T.,
Kubo
N.,
Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Research.
2015;
35
(10)
:
5369-76
.
PubMed Google Scholar -
Saito
R.,
Abe
H.,
Kunita
A.,
Yamashita
H.,
Seto
Y.,
Fukayama
M.,
Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Modern Pathology.
2017;
30
(3)
:
427-39
.
View Article PubMed Google Scholar -
Qing
Y.,
Li
Q.,
Ren
T.,
Xia
W.,
Peng
Y.,
Liu
G.L.,
Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Design, Development and Therapy.
2015;
9
:
901-9
.
View Article PubMed Google Scholar -
Eto
S.,
Yoshikawa
K.,
Nishi
M.,
Higashijima
J.,
Tokunaga
T.,
Nakao
T.,
Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer.
2016;
19
(2)
:
466-71
.
View Article PubMed Google Scholar -
Wu
P.,
Wu
D.,
Li
L.,
Chai
Y.,
Huang
J.,
PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One.
2015;
10
(6)
:
e0131403
.
View Article PubMed Google Scholar -
Xu
F.,
Feng
G.,
Zhao
H.,
Liu
F.,
Xu
L.,
Wang
Q.,
Clinicopathologic Significance and Prognostic Value of B7 Homolog 1 in Gastric Cancer: A Systematic Review and Meta-Analysis. Medicine.
2015;
94
(43)
:
e1911
.
View Article PubMed Google Scholar -
Liu
Y.X.,
Wang
X.S.,
Wang
Y.F.,
Hu
X.C.,
Yan
J.Q.,
Zhang
Y.L.,
Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. OncoTargets and Therapy.
2016;
9
:
2649-54
.
PubMed Google Scholar -
Schoemig-Markiefka
B.,
Eschbach
J.,
Scheel
A.H.,
Pamuk
A.,
Rueschoff
J.,
Zander
T.,
Optimized PD-L1 scoring of gastric cancer. Gastric Cancer.
2021;
24
(5)
:
1115-22
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 7 (2022)
Page No.: 5130-5139
Published on: 2022-07-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2976 times
- PDF downloaded - 653 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress




