Abstract
Left ventricular noncompaction, or noncompaction cardiomyopathy, is a rare congenital cardiomyopathy caused by the failure of the myocardial compaction. It has distinct morphological characteristics in the ventricular cavity and main cclinical manifestations are heart failure, arrhythmia, and thromboembolic complications. This paper reports on a patient who was admitted to the hospital with a diagnosis of acute myocardial infarction and severe left ventricular systolic dysfunction. During the patient's assessment and investigation, left ventricular noncompaction cardiomyopathy was diagnosed. In this literature review, we discuss the diagnostic criteria and the treatment of these patients.
Introduction
Noncompaction cardiomyopathy (NCC) is a rare disorder of myocardial morphogenesis that is characterized by excessive ventricular trabeculations and deep intratrabecular recesses. It has been categorized as “unclassified cardiomyopathy” by the European Society of Cardiology1. Previously, diagnosis of non-compaction cardiomyopathy was mainly based on 2D echocardiography, but many other methods (e.g., magnetic resonance imaging (MRI), computerized tomography) are currently being used for diagnosis confirmation. This disease may exist with or without other morphogenic or functional cardiac abnormalities. Here, we describe a case of NCC associated with complex coronary artery disease.
CASE REPORT
A male patient, 51-year-old, was admitted to the hospital because of shortness of breath during exertion. One month before admission, the patient began to experience difficulty in breathing after walking about 100 meters or climbing about 2 floors, as well as paroxysmal nocturnal dyspnoea. Additionally, the patient had no cough, fever, or chest pain. His early history has not recorded any cardiovascular disease, but has recorded hepatitis B and a history of smoking more than 20 packs per year. Clinical examination recorded mild dyspnea in the patient while lying down; heart rate was 90 bpm, blood pressure was 90/60 mmHg, and there were crackle rales at the base of two lungs. The electrocardiogram showed an incomplete left bundle branch block, negative T wave in V2 to V6, DI, and VL, suggesting myocardial ischemia (Figure 1). The electrocardiographic holter detected a short ventricular tachycardia. The echocardiography recorded dilatation of the left side of the heart (left ventricular end-diastolic diameter of 72 mm, left atrium diameter of 45 mm), akinesis of the apex, severe dyskinesis of the anterior, lateral, and septoanterior walls with severe ejection fraction (EF) decrease (28% Simpson 4C and 2C); the apex and mid-ventricle had many trabeculations, also called the noncompacted part, which was more than two-fold thicker than the normalcompacted part, with Doppler recording flow between trabeculations.
The MRI confirmed the diagnosis of myocardial noncompaction at the apex and the lateral wall of the left ventricle (LV). Examination also revealed myocardial necrosis in the distribution area of the left anterial descending (LAD) and left circumflex (LCx) arteries, as well as subendocardial perfusion defects (Figure 2).
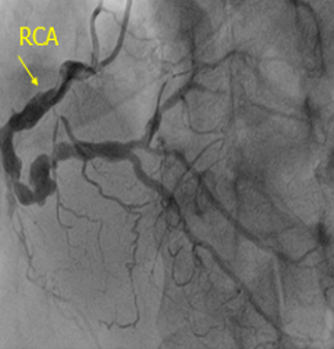
The coronary angiogram recorded total occlusion of the distal left main (LM) coronary artery, occlusion of the proximal LAD and LCx arteries with Rentrop II collaterals from the opposite side, and 90% stenosis of the right coronary artery (RCA) (Figure 3). Syntax score and STS score was 85 and 3.6%, respectively. The patient was transferred to have a coronary artery bypass surgery procedure and discharged after two weeks.
After 40 days, echocardiography showed that the left ventricular systolic function improved slightly, with EF going from 28% to 30% (Simpson 4C). For personal reasons, the patient refused to install an ICD. Despite this, he has reported feeling better and has had mild symptoms equivalent to the NYHA I level and is responding well to oral medical treatment for the LV dysfunction.
DISCUSSION
In the initial case series2, the median age at diagnosis of isolated noncompaction was 7 years (11 months to 22 years). In the largest series of these patients3, 4, the prevalence of patients investigated by echocardiography test was 0.014 – 1.3%, with prevalence expected to be 3 — 4% greater in HF patients. The true prevalence is not clear because correct diagnosis is often missed or delayed due to lack of knowledge on this disease and its similarity to other myocardial and endocardial diseases. The clinical case we described was 45 years old, with symptoms of left HF.
According to the literature, NCC has been reported to be familial recurrence. In children, the cause is mitochondrial gene transformation on the autosomes and the sex chromosomes. In adults, there is a genetic predominance of autosomes, while the form of sex chromosomes is rare. Mutations have been described in relation to many genes, such as TAZ/G4.5, ZASP/LPB3, DTNA, LMNA, and sarcomere protein-coding genes (MYH7, ACTC, 5NNT2). These genes have also been found to be involved in hypertrophic and dilated cardiomyopathy5, 6.
First described by Chin and colleagues as "porous cardiomyopathy," the cause of this disease is believed to be a failure of the compaction process of trabeculations that occurs between the fifth week and eighth week of the embryo, leading to the existence of long trabeculations and deep intratrabecular recesses that communicate with the left ventricular cavity7. Normally, the compaction process progresses from the epicardium to the endocardium, from the septum to the free wall, and from the base to the apex in the LV. Moreover, it is more often seen in the LV than in the right ventricle. Therefore, apical noncompaction is the most common type and is also considered one of the diagnostic criteria. At present, there has been no consensus guiding diagnosis, so Jenny's diagnostic criterion is the most widely used. This echocardiographic criterion consists of a two-layer structure with a thinner compacted layer and an inner noncompacted layer at a > 2:1 ratio of noncompacted to compacted myocardium, which is measured at end systolic phase in a parasternal short-axis vie, with blood flowing between the LV cavity and the recesses, and without secondary causes of increased trabeculations8. However, the right ventricular apex was involved in up to 41% of patients. One patient in Brazil was misdiagnosed with NCC when he was submitted to the hospital for the first time due to coronary disease, even undergoing coronary intervention. It was not until three years later, upon his second resubmission, that the echocardiography then revealed abnormal trabeculations9. In order to minimize the misdiagnosis of NCC, some other imaging methods are being applied, such as 3D echocardiography, magnetic resonance imaging, etc. Of these, cardiac magnetic resonance is considered as a tool for confirming diagnosis. Using an MRI, a previous study showed that a trabeculated LV myocardial volume above 35% of the total one is also diagnostic for NNC, with a high specificity of 89.7% and a sensitivity of 66.1%10. In our case, diagnosis of NCC was confirmed.
Most patients have no symptoms when diagnosed. However, clinical manifestations vary between patients. They may have HF (50%), arrhythmias (41%, including atrial fibrillation, ventricular tachycardia, bradycardia, branch block, and sudden death), or systemic thromboembolism (24%)11. Ventricular dysfunction may be systolic or diastolic. Although the mechanism of HF associated with NCC is unclear, it has been suggested that myocardial ischemia may play an important role. This eclipse is supported by subendocardial perfusion deficits that have been observed in cardiac MRI and PET7. The patient presented here is one of a few MRI-documented cases reported with subendocardial perfusion deficits, which will result in subendocardial fibrosis and necrosis associated with NCC. In this study's patient, these deficits were detected at the apical subendocardial cells through delayed enhancement by MRI (Figure 2). In order to explain these atherosclerotic-unrelated perfusion deficits, some authors considered that an overly thick subendocardial non-compacted layer may be the main cause restricting the endothelial myocardium from contacting the epicardial coronary arterial system, thereby causing ischemia. In this study’s case, it was difficult to determine the exact cause of HF, possibly due to a combination of atherosclerotic coronary artery disease and endothelial ischemia in NCC. This seems to be appropriate, as our patient with severe coronary artery disease was able to remain asymptomatic until middle age, indicating that he had been adapted to ischemia before. However, heart failure may likely be the result of this case of severe coronary disease based on the fact that the systolic function increased after the CABG12. Further data is needed to clarify this.
Although the relationship between NCC and coronary artery disease is not well understood, a number of articles have reported an association between NCC and acute myocardial infarction. A coronary angiography study in NCC subjects showed that 29% of patients had significant coronary artery disease13. Myocardial ischemia manifesting as a symptom of the NCC is thought to be caused by coronary dysfunction. A previous study concluded that coronary reserve decreases in NCC patients in both compacted and noncompacted cardiac muscles, including in the normal epicardial coronary artery system11.
Treatment of NCC so far has mainly focused on solving the symptoms. Patients with HF are treated with optimal medical treatment of ACE inhibitors or neprilysin, beta blockers, aldosterone antagonists, etc. Long-term anticoagulant therapy is suggested for patients with an EF less than 40%, a history of thrombotic embolism, or atrial fibrillation. In asymptomatic patients, the use of antiplatelet agents with aspirin has been recommended by some authors. Due to the high frequency of malignant arrhythmias, patients with NCC should be monitored with electrocardiograms for 24 hours at least once per year and consider setting vibration vibrators or investigating and treating by electric methods. End-stage patients need to be in the heart transplantation system. Additionally, patients are advised to avoid playing antagonistic sports and screen for the disease in direct relatives. In the case of our patient, we recorded a short ventricular tachycardia during hospitalization and recommended a vibrating pacemaker. However, the patient refused due to personal reasons. Fortunately, until now (six months after discharge), the patient has not complained of symptoms, such as palpitations, fainting, shortness of breath, etc., and HF status has remained stable at NYHA I.
The prognosis of NCC has improved in recent years due to the update and effectiveness of new drugs for the treatment of HF, new anticoagulants, and ICD. The three-year survival rate in patients with NCC was significantly higher (85% compared to 83%). Asymptomatic patients have even been considered for better prognosis14. In addition to optimal medical treatment, recommendation of a permanent pacemaker for prevention of secondary sudden death in cases of VT/VF and heart transplantation should be considered, if possible.
CONCLUSION
This clinical case illustrates the diversity in the manifestations of the disease and emphasizes a better screening of ultrasounds of patients with dilated cardiomyopathy and reduced EF, as well as a need for better guidelines on this particular pathological diagnosis and treatment.
Abbreviations
ACE: angiotensin-converting enzyme, ARB: angiotensin receptor blockers, CABG: coronary artery bypass grafting, HF: heart failure, IABP: intra-aortic balloon pump, ICD: implantable cardioverter defibrillator, NYHA: New York heart association, PET: positron emission tomography, STS: society of thoracic surgeons
Acknowledgments
Phuong L U Tran, MD (Cho Ray hospital) were involved in performing the second echocardiography.
Author’s contributions
Nghia T N, MD: Conceptualization; Writing - original draft; Formal analysis; Critical Review
My H T N, MD: Writing - original draft; Manuscrift editing; Critical Review
All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Following information must be provided in order for this form to be processed accurately. Patients have the right to refuse to sign this consent form; refusal to sign this form will not affect the care in any way.
"I hereby give my consent for images or other clinical information relating to my case to be reported in a medical publication. I understand that my name and initials will not be published and that efforts will be made to conceal my identity, but that anonymity cannot be guaranteed. I understand that the material may be published in a journal, website or other form of publication. At a result, I understand that the material may be seen by the general public. I understand that the material may be included in medical books."
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Elliott
P.,
Andersson
B.,
Arbustini
E.,
Classification of the cardiomyopathies: a position statement from the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J.
2008;
29
(2)
:
270-276
.
View Article PubMed Google Scholar -
Ichida
F.,
Left ventricular noncompaction. Circulation Journal.
2009;
73
(1)
:
19-26
.
View Article PubMed Google Scholar -
Oechslin
E.N.,
Attenhofer Jost
C.H.,
Rojas
J.R.,
Kaufmann
P.A.,
Jenni
R.,
Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. Journal of the American College of Cardiology.
2000;
36
(2)
:
493-500
.
View Article PubMed Google Scholar -
Goud
A.,
Padmanabhan
S.,
A rare form of cardiomyopathy: left ventricular non-compaction cardiomyopathy. Journal of Community Hospital Internal Medicine Perspectives.
2016;
6
(1)
:
29888
.
View Article PubMed Google Scholar -
Stöllberger
C.,
Finsterer
J.,
Blazek
G.,
Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. The American Journal of Cardiology.
2002;
90
(8)
:
899-902
.
View Article PubMed Google Scholar -
Zambrano
E.,
Marshalko
S.J.,
Jaffe
C.C.,
Hui
P.,
Isolated noncompaction of the ventricular myocardium: clinical and molecular aspects of a rare cardiomyopathy. Laboratory Investigation.
2002;
82
(2)
:
117-22
.
View Article PubMed Google Scholar -
Ichida
F.,
Hamamichi
Y.,
Miyawaki
T.,
Ono
Y.,
Kamiya
T.,
Akagi
T.,
Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. Journal of the American College of Cardiology.
1999;
34
(1)
:
233-40
.
View Article PubMed Google Scholar -
Kohli
S.K.,
Pantazis
A.A.,
Shah
J.S.,
Adeyemi
B.,
Jackson
G.,
McKenna
W.J.,
Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria?. European Heart Journal.
2008;
29
(1)
:
89-95
.
View Article PubMed Google Scholar -
Correia
E.,
Santos
L. F.,
Rodrigues
B.,
Gama
P.,
Cabral
C.,
Santos
O.,
Noncompaction of the myocardium in a patient with acute myocardial infarction. Arq. Bras. Cardiol. .
2010;
94
(5)
:
e62-e64
.
View Article Google Scholar -
Choi
Y.,
Kim
S.M.,
Lee
S.C.,
Chang
S.A.,
Jang
S.Y.,
Choe
Y.H.,
Quantification of left ventricular trabeculae using cardiovascular magnetic resonance for the diagnosis of left ventricular non-compaction: evaluation of trabecular volume and refined semi-quantitative criteria. Journal of Cardiovascular Magnetic Resonance.
2016;
18
(1)
:
24
.
View Article PubMed Google Scholar -
Jenni
R.,
Wyss
C.A.,
Oechslin
E.N.,
Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol.
2002;
39
(3)
:
450-454
.
View Article PubMed Google Scholar -
Emami
M.,
Banifatemeh
S.A.,
Behnamfar
Z.,
Non-compaction with Coronary Artery Disease; a Case Report. J. Biol. Today's World.
2014;
3
(6)
:
133-135
.
-
Aras
D.,
Tufekcioglu
O.,
Ergun
K.,
Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail.
2006;
12
(9)
:
726-733
.
View Article PubMed Google Scholar -
Oechslin
E.N.,
Attenhofer Jost
C.H.,
Rojas
J.R.,
Kaufmann
P.A.,
Jenni
R.,
Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. Journal of the American College of Cardiology.
2000;
36
(2)
:
493-500
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 9 No 7 (2022)
Page No.: 5191-5195
Published on: 2022-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2189 times
- PDF downloaded - 693 times
- XML downloaded - 0 times
 Biomedpress
Biomedpress




