Concise review: 3D cell culture systems for anticancer drug screening
Abstract
Three-dimensional (3D) cultures are becoming increasingly popular due to their ability to mimic tissue-like structures more effectively than monolayer cultures. In cancer research, the natural tumor characteristics and architecture are more closely mimicked by 3D cell models. Thus, 3D cell cultures are more promising and suitable models, particularly for in vitro drug screening to predict in vivo efficacy. Different methods have been developed to create 3D cell culture systems for research application. This review will introduce and discuss 3D cell culture methods most popularly used in drug screening. The potential applications of these systems in anticancer drug screening will also be discussed.
Introduction
Cancer is one of leading causes of death worldwide with 14 million new cases and 8.2 million deaths in 2012 (2014). Numerous efforts have been aimed at finding new and more effective ways to treat cancer. Among these strategies is screening of anticancer drugs. Standard screening has typically been evaluated in animal models. However, some results have shown that animal experiments do not always predict clinical outcome in humans, especially with regard to toxicity assessments Knight, 2008. Moreover, the use of animals for research is often restricted due to ethical concerns Festing, 2007. In light of these issues, an in vitro cell-based model is great alternative, minimizing the need for and number of animal experiments. 2D cell culture was the first procedure established for cell-based screening assays. Although 2D cell culture methods are simple, quick and cost-effective to set up, and have been widely investigated, there remain many disadvantages. The primary disadvantage of a 2D system is that it does not mimic an actual 3D tumor and is not biologically relevant Carrie J. Lovitt, 2014. Cells in the in vivoenvironment usually interact with neighboring cells and the extracellular matrix (ECM); however, 2D cell models cannot recapitulate those characteristics. Thus, a 2D culture model may be starkly different from an actual growing tumor with regards to cell morphology, cell proliferation, and gene and protein expression (Edmondson et al., 2014). As a result, only 10% of the drugs passed through in vitro testing have had a positive effect in the clinic, or led to drug approval. The percentage of anticancer drugs which have shown clinical efficacy is even lower, at about 5% (Westhouse, 2010). The high rate failure in the clinical testing phase is a waste of time and money. Therefore, it is important to identify promising in vitro culture models for evaluating drug efficacy in the early stages of drug discovery and development Wong et al., 2012. Given the advantages of 3D versus 2D cell culture models, 3D cell culture techniques garnered increasing attention. The number of publications related to 3D cell cultures have rapidly increased in the last decade- from 7 publications in 1992 to 421 in 2013 Ferro et al., 2014Ravi et al., 2015. The 3D cell culture systems allow cell-based assays to be more physiologically relevant, particularly since cell behavior in 3D culture is much more similar to that of cells in in vivo tissues. In 3D models, cell-cell and cell- ECM interactions are maintained, such that cell morphology, proliferation, differentiation, migration, apoptosis, gene expression and protein expression are comparable to those of cells in vivoEdmondson et al., 2014.
Why 3d culture?
Cell-based assays play a critical role in anticancer drug screening. Traditionally, 2D cell culture was widely used in cancer drug discovery. However, a large number of drugs reported to have strong anticancer effect in 2D cell culture models failed in clinical tests Xu and Burg, 2007. In 2011, although approximately 900 antineoplastic agents had passed through cell-based assay testing, only 12 were approved by the FDA after clinical testing America, 2011Kantarjian et al., 2013.
In recent years, the potential and critical role of 3D cultures in cancer research have gained greater interest. Through the use of sophisticated 3D multicellular tumor spheroid (MCTS) systems, the microenvironment, phenotype and cellular heterogeneity of tumors are effectively represented Thoma et al., 2014. MCTS systems create a gradient of oxygen and nutrients from the outside of tumor spheroids to the core. Spheroids in MCTS systems are constructed with different zones of cells, including proliferating cells on the outside, quiescent viable cells in the middle, and necrotic cells at the inner core ( Figure 1 ), which realistically mimic in vivo tumors Ma et al., 2012. Many research studies have shown that the genotypic profile of cells in MCTS, versus cells grown in monolayer, are more similar to in vivo tumors Smith et al., 2012. Cells in 3D culture conditions were found to exhibit gene expression profiles different to those grown in monolayer Luca et al., 2013Myungjin Lee et al., 2013. This may be a primary reason as to why results of anticancer drug assessments using MCTS are more predictive of clinical efficacy than 2D cell assessments Carver et al., 2014.
Many antineoplastic agents have been reported to be less effective for cancer cells cultured in 3D than 2D Frankel et al., 2000Imamura et al., 2015Karlsson et al., 2012. The architectural structure of MCTS is the main reason for this difference. Firstly, the 3D structure of MCTS reduces the number of cancer cells exposed to anticancer agents; these drugs have more accessibility to cells in monolayer culture Carrie J. Lovitt, 2014. Secondly, the tightly adhered cells and ECM in MCTS can limit drug penetration Frankel et al., 2000. Moreover, the hypoxic core generates a G0- dormant cell population which is highly resistant to chemotherapy Imamura et al., 2015. Gene expression of cells cultured in 3D systems differs from that of cells in 2D monolayer; for instance, expression of genes related to chemoresistance has been found to vary from 3D versus 2D systems Lin and Chang, 2008. Studies in breast cancer Howes et al., 2014a and colon cancer Luca et al., 2013 have demonstrated decreased epidermal growth factor (EGFR) and human epidermal growth factor (HER) activation in cells cultured in 3D versus 2D. This could cause decreased sensitivity to anticancer drugs targeting EGFR and HE, and has been observed in 3D cell systems. On the other hand, some drugs show equal, or even greater, therapeutic effect in 3D models compared to 2D Hongisto et al., 2013Howes et al., 2007Pickl and Ries, 2009. The absence of a hypoxic, necrotic core in 2D culture models makes cells more resistant to antineoplastic agents, which are effectively activated by hypoxic conditions of 3D tumors; tirapazamine (TPZ) is an example of this kind of drug Tung et al., 2011. Given that 3D models not only mimic tumor architecture but mimic similar environmental challenges, these models are great and conservative systems to study candidate drug.
Although MCTS is still an in vitro model, its similarity to an in vivo tumor environment allows for a more accurate model to study drug efficacy while minimizing the cast of failed clinical trials.
Platforms of 3d cell culture systems used for anticancer drug screening
Due to the advantages of 3D culture systems, there have been many studies focused on the development and optimization of 3D cell culture technologies. Up until now, there have been several types of 3D culture modeis, same of which have been used for anticancer drug screening.
Liquid overlay culture
Liquid overlay culture (LOC) is the simplest method of 3D cell culture Enmon et aI., 2001. To generate models, cell culture plates or flasks are covered with a thin layer of inert substrates, such as agarVinci et al., 2012, agarose Friedrich et aI., 2009, polyHEMAFriedrich et aI., 2007 or Matrigel C. S. SHIN 2013. By preventing matrix deposition, LOC easily promotes 3D aggregates or sph eroidsCa risson and Yuhas, 1984. This technique is low cost and highly reproducible without requirement of sophisticated equipment Costa et aI., 2014. Different cell types can be co-cultured with this method Metzger et a1.. However, it is difficult to monitor the number and size of formed spheroids Lin and Chang, 2008.
Ultra-low attachment plates have been developed as the commercial product of the liquid overlay technique, bypassing the requirement for manual coating. Dishes are designed with a layer of hydrophilic polymer inside, which prevents cells from attaching to the surface. This technique can overcome the limit of culture in gel, has the potential to produce one spheroid per well, and is suitable for medium-throughput screening Thoma et al., 2014.
Hanging drop
The hanging drop technique was first developed by Johannes Holtfreter in 1944 for culivating embryonic stem cells. lt has also become the foundation of the non-scaffold method for the multicellular spheroid generalion. In the beginning, the petri dish lid was used to generate spheroids by dropping a small volume of cell suspension (15- 30 μL) onto the lid and then inverting it. Due to surface tension, droplets were maintained and cells in the drop lets spontaneously aggregated to form spheroids Lin and Chang, 2005. Today, there are many types of commercial devices designed for hanging drop cultures ( Figure 2 ).
This technique has many advantages, including being cost-effective, easy to generate one spheroid per well, and easy to control the size of spheroids. Moreover, different cell types can be cocultured and generated into spheroids at high-throughput using liquid handling systems Hsiao et al., 2012Kelm et al., 2003Pham, 2015Yip and Cho, 2013. However, it is difficult to maintain spheroids and change the medium due to the limited volume of drop lets Mehta et al., 2012.
Microtechnology
In the last few years, microtechnologies have attracted the attention of scientists, particularly with regard to the use of microtechniques to generate 3D cell models Hirschhaeuser et al., 2010.
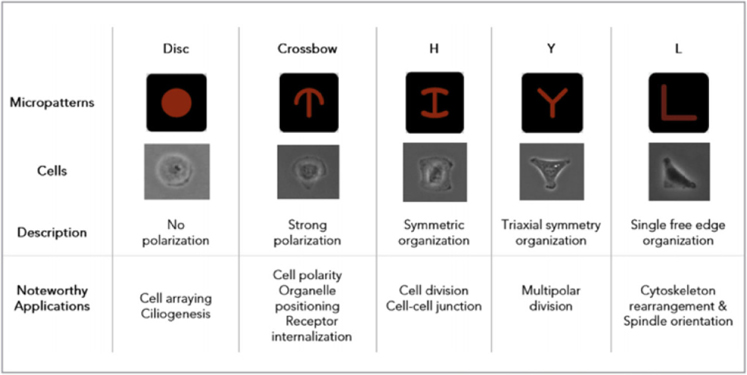
The photolithography technique is one exampleand used to create micropattem surface plates with special surfaces, including attaching and non-attaching areas. Seeded cells are guided to grow and farm 3D structureson the adhesion islands. The size and shape of spheroids rely on the design of the attachment sites ( Figure 3 ) Degot et al., 2010.
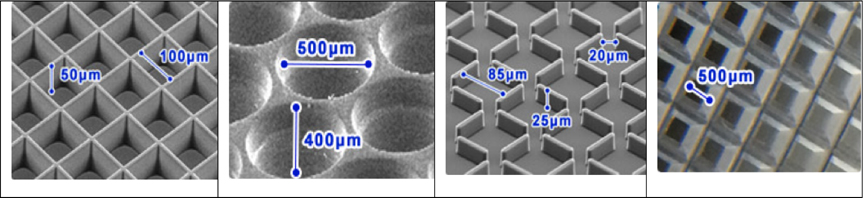
Microwell plates are designed with the bottom containing a large number of microsize chambers, which vary in shape, e.g. round, square, honeycomb, slit and multiple poresLarson, 2015 ( Figure 4 ). Under gravity and hydrodynamic forces, cells are located in tiny wells and then concentrated to form 30 structure with dimensions and geometry specific to each type of microwell Karp et al., 2007.
Microtechnologies, including microwells and micropattern surfaces, are promising for producing mass production of controlled sized spheroids. It is possible to co-culture different type of cells through the requirement of special and expensive equipment Lin and Chang, 2008.
Bioreactor
When the important role of 3D cultures in testing chemical effects of anticancer drugs was discovered, scale-up screening from laboratory to industrial scale became a critical next step. Bioreactors became part of the standard process for spheroid generation as they provided greater production control and reproducibility Ou and Hosseinkhani, 2014. In a typical process, spheroids are formed in bioreactors via continuous moving fluid (Breslin and O'Driscoll, 2013). The dynamic culture condition is mainly created by stirring (spinner flask) or rotating (NASA rotating wall vessel) c. S. SHIN 2013.
The modern glass spinner flask was first developed by W.F. McLimans in 1957 Mc et al., 1957. Cell suspension was contained in flasks, which were designed with two arms and could be opened for gas exchange; a stir bar was used for stirring the fluid Delphine Antoni 2015 ( Figure 5a ). In 1990, rotating wall vessels (RWVs) were made for cell culture by NASA (National Aeronautics and Space Administration) K. C. O'Connor', 2013. RWVs are constructed of an inner cylinder, a chamber of rotating concentric cylinders for growing cells, and a membrane for gas exchange Rauh et al., 2011 ( Figure 5b ). The low shear environment of RWVs creates larger sized spheroids than spinner flasks Lelkes and Cherian, 1995. HepG2 spheroids formed in RWVs reach 100 μm in diameter after 72 h of culture and up to 1 mm in diameter after long-term culture Chang and Hughes-Fulford, 2009.
Bioreactors are labor-intensive due to their ability to produce a large number of spheroids Tostoes et al., 2012. However, the created spheroids are usually heterogeneous in size and cell population Mehta et al., 2012. Therefore, a manual selection would be required afterward to select suitably sized spheroids for re-plating onto dishes for drug screening assays, if the similarity of spheroid size is required Breslin and O'Driscoll, 2013. Although generation of spheroids via bioreactors requires expensive instruments Kim et al., 2004 and high quality of medium, the advantages of bioreactors for long-term culture is undeniable Ebrahimkhani et al., 2014.
Applications in anticancer drug screening
Cell culture systems have long been a foundation for testing and comparing the cytotoxicity and pharmacodynamics of anticancer drug candidates. Even now, many results from 3D cell culture have consistently stressed the importance of these models in drug screening. Research by Jayme L. Horning et al., published in 2008, indicated that 3D MCF7 cells were more resistant to many popular anticancer drugs (e.g. doxorubicin, paclitaxel and tamoxifen) compared with MCF7 cells cultured in monolayer. Using polymeric microparticle surfaces to create 3D tumors, they found that 2D MCF7 cells were significantly more sensitive to these drugs than 3D MCF7 cells, with a 12- to 23- fold disparity in the IC50 values. The study also showed that the sum of collagen in the 3D model was 2 times greater than that of 2D condition and the expression of many genes were different, possibly accounting for the difference in responses to the drugs Horning et al., 2008. Vesa Hongisto et al. suggested in their 2013 studies that 3D cell models can effectively replace traditional 2D cell monolayers and that with regard to screening of drug compounds, 3D models provide better comparability to clinical results. In their study, 102 compounds were tested on JIMT1 breast cancer cells. Results showed that JIMT1 cells were significantly more sensitive to 63 compounds when cultured on Matrigel as compared to 2D condition Hongisto et al., 2013. Using 96-well roundbottom ultra-low attachment plates to create 3D cancer tumors, Amy L. Howes et al. showed, from their studies in 2014, that 3D BT-474 cells were more sensitive to lapatinib, gefitinib, vinblastine and vinorelbine than 3D MCF-10A cells. The authors also found that microtubule-targeting agents and epidermal growth factor receptor (EGFR) inhibitors are two classes of compounds to have selective effects on cancer cells in 3D culture Howes et al., 2014b. Work by Yukie Yoshii et al., published in 2016, on human colon cancer HCT116 cell line demonstrated that regorafenib was most effective on 3D HCT116- RFP cells among 8 drugs tested (capecitabine, bevacizumab, irinotecan, cetuximab, 5-fluorouracil (5- FU), panitumumab, oxaliplatin and regorafenib). Based on their 3D culture studies, the authors were able to demonstrate effective and non-effective drugs for colon cancer treatment Yoshii et al., 2016.
Conclusion
Anticancer drug screening is an important component in the fight against cancer. Several 3D cell culture systems have been developed as suitable platforms for drug screening and are serve as more reliable models for in vitro testing, compared to 2D, given that MCTS have greater structural similarity and cellular zone components to in vivo tumors. The 3D model systems should provide more accurate results for prediction of clinical outcome. Tremendous efforts have been made to establish various 3D cell culture systems. It is important for researchers to look carefully at the advantages and disadvantages of each to find the most suitable system for their studies. However, all the 3D systems can be utilized for cancer research, particularly for testing of new anticancer agents.
References
-
P.R.a.M.o.
America.
Medicines in Development for Cancer. 2011
.
-
S.
Breslin,
L.
O’Driscoll.
Three-dimensional cel culture: the missing link in drug discovery. Drug discovery today.
2013;
18
:
240-249
.
-
B.K.
C. S. SHIN,
K. PARK A. PANITCH
B. HAN.
3D cancer tumor models for evaluating chemotherapeutic efficacy. 2013
.
-
J.
Carlsson,
J.M.
Yuhas.
Liquid-overlay culture of cellular spheroids. ecent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le.
1984;
cancer95
:
1-23
.
-
T.B.S.a.V.M.A.
Carrie J. Lovitt.
Advanced Cell Culture Techniques for Cancer Drug Discovery. 2014
.
-
K.
Carver,
X.
Ming,
R.L.
Juliano.
Multicellular Tumor Spheroids as a Model for Assessing Delivery of Oligonucleotides in Three Dimensions. Mol Ther Nucleic Acids.
2014;
3
:
e153
.
-
T.T.
Chang,
M.
Hughes-Fulford.
Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. issue engineering Part A.
2009;
15
:
559-567
.
-
E.C.
Costa,
V.M.
Gaspar,
P.
Coutinho,
I.J.
Correia.
Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models. Biotechnology and bioengineering.
2014;
111
:
1672-1685
.
-
S.
Degot,
M.
Auzan,
V.
Chapuis,
A.
Béghin,
A.
Chadeyras,
C.
Nelep,
M.L.
Calvo-Muñoz,
J.
Young,
F.
Chatelain,
A.
Fuchs.
Improved Visualization and Quantitative Analysis of Drug Effects Using Micropatterned Cells. Journal of Visualized Experiments: JoVE.
2010
.
-
H.B.
Delphine Antoni,
Josset
Elodie,
Noel
Georges.
Three-Dimensional Cell Culture: A Breakthrough in Vivo. International journal of molecular sciences.
2015
.
-
M.R.
Ebrahimkhani,
J.A.S.
Neiman,
M.S.B.
Raredon,
D.J.
Hughes,
L.G.
Griffith.
Bioreactor Technologies to Support Liver Function In Vitro. Advanced drug delivery reviews.
2014;
0
:
132-157
.
-
R.
Edmondson,
J.J.
Broglie,
A.F.
Adcock,
L.
Yang.
Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay and Drug Development.
2014;
Technologies12
:
207-218
.
-
R.M. Jr.
Enmon,
K.C.
O’Connor,
D.J.
Lacks,
D.K.
Schwartz,
R.S.
Dotson.
Dynamics of spheroid self-assembly in liquid-overlay culture of DU 145 human prostate cancer cells. Biotechnology and bioengineering.
2001;
72
:
579-591
.
-
F.
Ferro,
C.
Shields Baheney,
R.
Spelat.
Three- Dimensional (3D) Cell Culture Conditions, Present and Future Improvements. Razavi Int J Med.
2014;
2
:
e17803
.
-
S.
Festing.
The ethics of animal research. Talking Point on the use of animals in scientific research.
2007;
8
:
526-530
.
-
A.
Frankel,
S.
Man,
P.
Elliott,
J.
Adams,
R.S.
Kerbel.
Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clinical cancer research: an official journal of the American Association for Cancer Research.
2000;
6
:
3719-3728
.
-
J.
Friedrich,
R.
Ebner,
L.A.
Kunz-Schughart.
Experimental anti-tumor therapy in 3-D: spheroids-old hat or new challenge?. International journal of radiation biology.
2007;
83
:
849-871
.
-
J.
Friedrich,
C.
Seidel,
R.
Ebner,
L.A.
Kunz-Schughart.
Spheroid-based drug screen: considerations and practical approach. Nature protocols.
2009;
4
:
309-324
.
-
F.
Hirschhaeuser,
H.
Menne,
C.
Dittfeld,
J.
West,
W.
Mueller- Klieser,
L.A.
Kunz-Schughart.
Multicellular tumor spheroids: an underestimated tool is catching up again. Journal of biotechnology.
2010;
148
:
3-15
.
-
V.
Hongisto,
S.
Jernstrom,
V.
Fey,
J.P.
Mpindi,
K.
Kleivi Sahlberg,
O.
Kallioniemi,
M.
Perala.
Highthroughput 3D screening reveals differences in drug sensitivities between culture models of JIMT1 breast cancer cells. PloS one.
2013;
8
:
e77232
.
-
J.L.
Horning,
S.K.
Sahoo,
S.
Vijayaraghavalu,
S.
Dimitrijevic,
J.K.
Vasir,
T.K.
Jain,
A.K.
Panda,
V.
Labhasetwar.
3-D tumor model for in vitro evaluation of anticancer drugs. Molecular pharmaceutics.
2008;
5
:
849-862
.
-
A.L.
Howes,
G.G.
Chiang,
E.S.
Lang,
C.B.
Ho,
G.
Powis,
K.
Vuori,
R.T.
Abraham.
The phosphatidylinositol 3- kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Molecular cancer therapeutics.
2007;
6
:
2505-2514
.
-
A.L.
Howes,
R.D.
Richardson,
D.
Finlay,
K.
Vuori.
3-Dimensional Culture Systems for Anti-Cancer Compound Profiling and High-Throughput Screening Reveal Increases in EGFR Inhibitor-Mediated Cytotoxicity Compared to Monolayer Culture Systems. PloS one.
2014a;
9
.
-
A.L.
Howes,
R.D.
Richardson,
D.
Finlay,
K.
Vuori.
3-Dimensional culture systems for anti-cancer compound profiling and high-throughput screening reveal increases in EGFR inhibitor-mediated cytotoxicity compared to monolayer culture systems. PloS one.
2014b;
9
:
e108283
.
-
A.Y.
Hsiao,
Y.C.
Tung,
X.
Qu,
L.R.
Patel,
K.J.
Pienta,
S.
Takayama.
384 hanging drop arrays give excellent Zfactors and allow versatile formation of co-culture spheroids. Biotechnology and bioengineering.
2012;
109
:
1293-1304
.
-
Y.
Imamura,
T.
Mukohara,
Y.
Shimono,
Y.
Funakoshi,
N.
Chayahara,
M.
Toyoda,
N.
Kiyota,
S.
Takao,
S.
Kono,
T.
Nakatsura.
Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncology reports.
2015;
33
:
1837-1843
.
-
T.L.P.
K. C. O’Connor’,
T. J. Goodwinft,
K. M. Francis’,
A. D. Andrews’,
G. F. Spauldingff.
Animal cell cultivation in the NASA roating wall vessel. 2013
.
-
H.
Karlsson,
M.
Fryknas,
R.
Larsson,
P.
Nygren.
Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Experimental cell research.
2012;
318
:
1577-1585
.
-
J.M.
Karp,
J.
Yeh,
G.
Eng,
J.
Fukuda,
J.
Blumling,
K.Y.
Suh,
J.
Cheng,
A.
Mahdavi,
J.
Borenstein,
R.
Langer.
Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab on a chip.
2007;
7
:
786-794
.
-
J.M.
Kelm,
N.E.
Timmins,
C.J.
Brown,
M.
Fussenegger,
L.K.
Nielsen.
Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and bioengineering.
2003;
83
:
173-180
.
-
J.B.
Kim,
R.
Stein,
M.J.
O’Hare.
Three-dimensional in vitro tissue culture models of breast cancer- a review. Breast cancer research and treatment.
2004;
85
:
281-291
.
-
A.
Knight.
Systematic reviews of animal experiments demonstrate poor contributions toward human healthcare. Reviews on recent clinical trials.
2008;
3
:
89-96
.
-
B.
Larson.
3D Cell Culture: A Review of Current Techniques. 2015
.
-
P.I.
Lelkes,
D. L.
Galvan,
G.
Thomas Hayman,
T. J.
Goodwin,
D. Y.
Chatman,
S.
Cherian,
R. M. G. Unsworth
Garcia.
Simulated microgravity conditions enhance differentiation of cultured PC12 cells towards the neuroendocrine phenotype. In Vitro Cell Dev Biol-Anim.
1998
.
-
R.Z.
Lin,
H.Y.
Chang.
Recent advances in threedimensional multicellular spheroid culture for biomedical research. Biotechnology journal.
2008;
3
:
1172-1184
.
-
A.C.
Luca,
S.
Mersch,
R.
Deenen,
S.
Schmidt,
I.
Messner,
K.L.
Schafer,
S.E.
Baldus,
W.
Huckenbeck,
R.P.
Piekorz,
W.T.
Knoefel.
Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PloS one.
2013;
8
:
e59689
.
-
H.L.
Ma,
Q.
Jiang,
S.
Han,
Y.
Wu,
J.
Cui Tomshine,
D.
Wang,
Y.
Gan,
G.
Zou,
X.J.
Liang.
Multicellular tumor spheroids as an in vivo-like tumor model for three-dimensional imaging of chemotherapeutic and nano material cellular penetration. Molecular imaging.
2012;
11
:
487-498
.
-
L.W.
Mc,
E.V.
Davis,
F.L.
Glover,
G.W.
Rake.
The submerged culture of mammalian cells; the spinner culture. Journal of immunology.
1957;
(Baltimore
:
Md: 1950) 79, 428-433
.
-
G.
Mehta,
A.Y.
Hsiao,
M.
Ingram,
G.D.
Luker,
S.
Takayama.
Opportunities and Challenges for use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. Journal of controlled release: official journal of the Controlled Release Society.
2012;
164
:
192-204
.
-
W.
Metzger,
D.
Sossong,
A.
Bächle,
N.
Pütz,
G.
Wennemuth,
T.
Pohlemann,
M.
Oberringer.
The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy.
;
13
:
1000-1012
.
-
J.
Myungjin Lee,
P.
Mhawech-Fauceglia,
N.
Lee,
L.
Cristina Parsanian,
Y.
Gail Lin,
S.
Andrew Gayther,
K.
Lawrenson.
A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab Invest.
2013;
93
:
528-542
.
-
K.L.
Ou,
H.
Hosseinkhani.
Development of 3D in vitro technology for medical applications. nternational journal of molecular sciences.
2014;
15
:
17938-17962
.
-
P.
Pham.
Breast Cancer Stem Cell Culture and Proliferation. In Breast Cancer Stem Cells & Therapy Resistance. Cham: Springer International Publishing.
2015;
:
41-55
.
-
M.
Pickl,
C.H.
Ries.
Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene.
2009;
28
:
461-468
.
-
J.
Rauh,
F.
Milan,
K.P.
Gunther,
M.
Stiehler.
Bioreactor systems for bone tissue engineering. issue engineering Part.
2011;
B
:
Reviews 17, 263-280
.
-
M.
Ravi,
V.
Paramesh,
S.R.
Kaviya,
E.
Anuradha,
F.D.
Solomon.
3D cell culture systems: advantages and applications. Journal of cellular physiology.
2015;
230
:
16-26
.
-
S.J.
Smith,
M.
Wilson,
J.H.
Ward,
C.V.
Rahman,
A.C.
Peet,
D.C.
Macarthur,
F.R.
Rose,
R.G.
Grundy,
R.
Rahman.
Recapitulation of tumor heterogeneity and molecular signatures in a 3D brain cancer model with decreased sensitivity to histone deacetylase inhibition. PloS one.
2012;
7
:
e52335
.
-
C.R.
Thoma,
M.
Zimmermann,
I.
Agarkova,
J.M.
Kelm,
W.
Krek.
3D cell culture systems modeling tumor growth determinants in cancer target discovery. Advanced drug delivery reviews.
2014;
69-70
:
29-41
.
-
R.M.
Tostoes,
S.B.
Leite,
M.
Serra,
J.
Jensen,
P.
Bjorquist,
M.J.
Carrondo,
C.
Brito,
P.M.
Alves.
Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology (Baltimore, Md).
2012;
55
:
1227-1236
.
-
Y.C.
Tung,
A.Y.
Hsiao,
S.G.
Allen,
Y.S.
Torisawa,
M.
Ho,
S.
Takayama.
High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst.
2011;
136
:
473-478
.
-
M.
Vinci,
S.
Gowan,
F.
Boxall,
L.
Patterson,
M.
Zimmermann,
W.
Court,
C.
Lomas,
M.
Mendiola,
D.
Hardisson,
S.A.
Eccles.
Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biology.
2012;
10
:
29
.
-
R.A.
Westhouse.
Safety assessment considerations and strategies for targeted small molecule cancer therapeutics in drug discovery. Toxicologic pathology.
2010;
38
:
165-168
.
-
C.C.
Wong,
K.W.
Cheng,
B.
Rigas.
Preclinical Predictors of Anticancer Drug Efficacy: Critical Assessment with Emphasis on Whether Nanomolar Potency Should Be Required of Candidate Agents. The Journal of Pharmacology and Experimental Therapeutics.
2012;
341
:
572-578
.
-
F.
Xu,
K.J.L.
Burg.
Three-dimensional polymeric systems for cancer cell studies. Cytotechnology.
2007;
54
:
135-143
.
-
D.
Yip,
C.H.
Cho.
A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochemical and biophysical research communications.
2013;
433
:
327-332
.
-
Y.
Yoshii,
T.
Furukawa,
H.
Aoyama,
N.
Adachi,
M.R.
Zhang,
H.
Wakizaka,
Y.
Fujibayashi,
T.
Saga.
Regorafenib as a potential adjuvant chemotherapy agent in disseminated small colon cancer: Drug selection outcome of a novel screening system using nanoimprinting 3-dimensional culture with HCT116-RFP cells. International journal of oncology.
2016;
48
:
1477-1484
.
-
H.M.
Kantarjian,
T.
Fojo,
M.
Mathisen,
L.A.
Zwelling.
Cancer drugs in the United States: Justum Pretium—the just price. Journal of Clinical Oncology.
2013;
31
:
3600-3604
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 05 (2016)
Page No.: 625-632
Published on: 2016-05-27
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6989 times
- Download PDF downloaded - 1401 times
- View Article downloaded - 8 times
 Biomedpress
Biomedpress