Investigation of In-vivo Anti-diarrheal and In-vitro Antihelminthic properties of Methanolic Leaves Extract of Dalbergia stipulacea Roxb
Abstract
Aim: This study is designed to identify and evaluate the pharmacological property of leaf extract of D. stipulacea, commonly known as Horoiludi in Bangladesh.
Materials and Methods: The anti-diarrheal activity was estimated using Galvez et al. method with a simple modification. The testing extract was compared to standard Loperamide (5 mg/kg, p.o.), while the extract had two different concentration 200 mg/kg and 400 mg/kg. On the other hand antihelminthic property of this plant was investigated using Panagrellus redivivus worms.
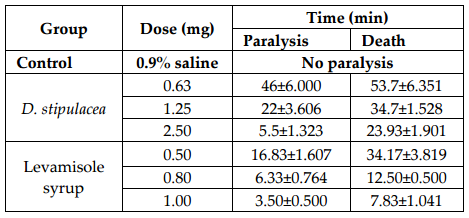
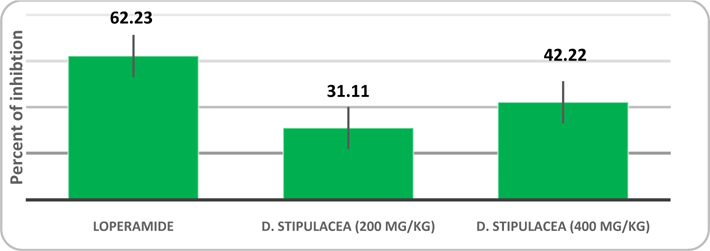
Result: At maximum concentration (400 mg/kg) it inhibited 42.22 % and loperamide was found to inhibit 62.23 %. The one-way ANOVA for this testing result in P < 0.0001, which means that the investigation result have significant importance in consideration of statistical analysis. The antihelminthic testing also results in a dose dependent manner. At highest dose (2.5 mg) the extract paralyzed experimental worm in 5.5 ± 1.323 min and it died in 23.93 ± 1.901 min.
Conclusion: Based on the data analysis obtained from the study, it can be concluded that the plant part has good anti-diarrheal activity as well as antihelminthic property. We need to identify its actual molecule through further processing.
Background
In developing countries, diarrhea is one of the most prominent reasons of death among infants. It has been approximated that diarrhea has caused death of 1.87 million children (0-5 years) globally and among them 73% are from only 15 countries which also include Bangladesh Boschi-Pinto et al., 2008. Parasitic helminthes affects animals and men and causes substantial privation and reduced growth in human development.
Helminthes mostly cause chronic diseases which are of debilitating nature. Perhaps they cause greater social and economic divestment and more morbidity among humans and animals than any individual group of parasites. Parasites which are immature do invade human beings through thegastrointestinal tract (GIT) or skin and usuallygrow into well differentiated adult worms having characteristic tissue distribution.
Anti-helminthics are drugs that may act locally to expel worms from the GIT or systemically to eradicate adult helminthes or development forms that invade organs and tissues AG, 1980. Intestinal helminths like AscarisLumbricoides (ascaris) andNecatorAmericanus and AncylostomaDuodenale (hookworm) are prevalent in Bangladesh in all age groups Martin et al., 1983. Ascaris has been related with VAD Hall et al., 1992Sivakumar and Reddy, 1975 and hookworm initiates blood loss which causes iron deficiency anaemia Jalal et al., 1998Pritchard et al., 1991. Several researchers reported that worm load and fecal egg count have a strong relation with the amount of blood loss and cause iron deficiency anemia CROMPTON, 2000Inoue et al., 2013Kuo et al., 2010Stoltzfus et al., 1997.
However, the burden of the worm usually depends on the iron stocks of the population Dreyfuss et al., 1996Stoltzfus et al., 1997. Several pharmacological activities were reported for various Dalbergia species. D. sissoo has anti-inflammatory Asif and Kumar, 2009, antipyretic Asif and Kumar, 2011, anthelmintic Hood et al., 2011, osteogenic Dixit et al., 2012 and anti-diarrheal Kalaskar et al., 2010 properties; Antiallergic and antioxidant activity was found in D. odorifera Cheng et al., 1998 D. paniculata contains antiinflammatory and antioxidant activity Ganga et al., 2012. Anti-ulcerogenic effect was found in D. monetaria (COTA et al., 1999). Anti-diarrheal effect was also evaluated in D. lancedaria Mujumdar et al., 2005.
After observing the reported anti-diarrheal and anthelmintic properties of Dalbergiaspecies, the study was undertaken to evaluate the methanolic leaf extract of D. stipulacea Roxb for its antidiarrheal and anthelmintic activity.
Materials and methods
Plant Material
The leaves of the Dalbergiastipulacea Roxbwere collected from hilly regional local forest of Chittagong District and identified and authenticated by a famous taxonomist of Bangladesh, Dr. Sheikh Bakhtiar Uddin, Associate Professor, Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh. Voucher Specimens were deposited in the National Herbarium, Bangladesh.
Extract Preparation
After cleaning, the leaves were taken and air dried for 10 days, and then kept in an oven at 45°C at 72 hours. 500gm of dried powder was cold extracted with methanol since methanol is the most common solvent for extracting most of the constituents present in herbal materials. Amber glass bottle was used for this purpose, which were kept at room temperature and allowed to stand for 5-7 days with occasional shaking and stirring. The combined extractwas filtered and evaporated to dryness using a rotary evaporator.A membrane pump was used to evacuate the extract in order toremove the residual solvent. The extract was finally freezedried (275 g) by using a Varian 801 LY-3- TT freeze-dryer (Varian, Lexington, MA, USA). The dry sample was stored at 4°C.
Chemicals and Reagents
All chemicals and solvents which were used in this study were of analytical grade and procured from Merck, Germany. Standard drug such as Loperamide (Brand: Imotil®) was purchased from Square Pharmaceuticals Limited, Bangladesh and Levamisole (Brand: Etrax®) was bought from ACI Limited, Bangladesh. Castor oil was purchased from WELL’S Health Care, Spain.
Experimental animals and organisms
Swiss Albino mice having weight 35-45g were obtained from the animal house of the international center for diarrheal disease and research, Bangladesh (icddr’b).The animals were housed under the standard laboratory conditions (relative humidity 55-65%), room temperature 23.0 ± 2.00C and 12h light: dark cycle). The animals were fed with standard diet and water. Appropriate measures were taken to minimize the discomfort of animals and all protocols for animal experiment were followed by the institutional animal ethical committee and the ethical guidelines issued by the International Association for the Study of Pain Zimmermann, 1983. The free living nematode Panagrellusredivivus (sour paste nematode) is known to many aquarium enthusiasts and fish keepers as the micro worm or black worm were collected from Aquarium fish center, Chittagong, Bangladesh which is used as fish food.
Preliminary phytochemical Screening
The methanolic leaf extract of D. stipulacea Roxb was screened for the availability of various bioactive phytochemical compounds. Specific qualitative tests were performed to detect bioactive compounds of pharmacological importance through standard methods. 1 g of the methanolic leaf extract of D. stipulacea Roxb was dissolved in 100 ml of methanol and was subjected to preliminary phytochemical screenings for determining nature of phytoconstituents Brain and Turner, 1975Harborne, 1998IL,1983Kokate, 2001Paech and Tracey, 1955.
Test for Carbohydrates
2 ml of extracts and 2 drops of molisch’s reagent was mixed and shaken well in a test tube. Then 2 ml of conc. H2SO4 was added to that mixture. A reddish violet colour ring appeared at the junction of two layers immediately indicated the presence of observed.
Test for Cholesterol
2 ml of extracts and chloroform was added in dry test tube. Then 10 drops of acetic anhydride and 2 to 3 drops of concentrated H2SO4 was added. A red rose colour changed to blue green colour.
Test for alkaloids
A few drops of dilute HCl was added to extract (2 ml) for acidification. 1 ml of Dragendroff’s reagent was added to that mixture and orange color precipitate change to red, indicating the presence of alkaloids.
Test for tannins
A few drops of 10% lead acetate was added to extract (2 ml), white color sedimentation appeared, indicates the presence of tannin.
Test for Cardiac glycosides (Keller-Killani test)
5 ml of extract was mixed with 2 ml of glacial acetic acid containing one drop of ferric chloride (FeCl3) solution, followed by the addition of 1 ml concentrated sulphuric acid. Brown ring was formed at the interface which indicated the presence of deoxysugar of cardenoloides. A violet ring may appear beneath the brown ring, while in the acetic acid layer, a greenish ring may also form just gradually throughout the layer.
Test for saponins
1 ml extract was mixed up with 9 ml of distilled water in tube and shaken comprehensively. The mixture was allowed to stand for about 10-15 mins. Appearance of stable foam indicates the presence of saponins.
Test for Phenols
1ml of extract of sample and 2ml of distilled water followed by a few drops of 10% aqueous ferric chloride solution were added in a dry test tube. Formation of blue or green colour indicated the presence of phenols.
Test for steroids
2 ml of plant extract was taken with 10 ml of chloroform and addition of 1 ml acetic anhydride and 2 ml of sulphuric acid. Notable bluish green color at the junction indicates the presence of steroids.
Test for Resins
One ml of extract was treated with few drops of acetic anhydride solution followed by one ml of concentrated H2SO4. Resins give colouration ranging from orange to yellow.
In vivoanti-diarrheal activity test: Acute toxicity test
Acute toxicity test for the extract of D. stipulacea Roxbwas carried out following the method of Lorke to evaluate any possible toxicity Joshi et al., 2007Jothy et al., 2011 OECD. Different doses of methanolic leaves extract were injected intra-peritonealy into groups of 12 Swiss albino mice. The injected maximum dose was 600 mg/kg. At the same time, the control group only received distilled water. The number of deaths of experimental mice was counted at 48 h after treatment.
Methodology
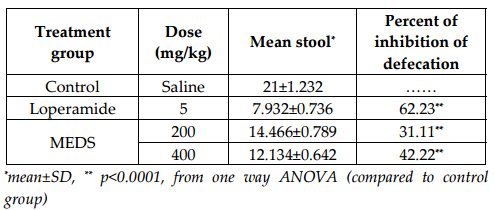
The method which was followed by Galvez et al. for antidiarrheal activity test was modified to suit the experimental need Galvez et al., 1993Galvez et al., 1991. Experimental albino mice were fasted 24 h before the test with free access to water and divided into 4 groups of 4 animals each. Diarrhea was induced by administering 0.5ml of castor oil orally. Group A treated as control and received distilled water 0.5 mL, per oral (p.o.), Group B received standard drug (Loperamide 5mg/ kg body weight, p.o.) and Group C and Group D received methanolic leaf extract of D. stipulacea Roxb (200mg/kg and 400 mg/kg respectively). 30 min later castor oil was administered (0.5 mL) to the mice. Each animal was placed in individual cage, where the floor of cage was lined by blotting paper. The floor lining was changed every hour interval. The consistency of the faecal matter and the number of both the wet and the dry diarrheal droppings were counted every hour up to 4 hours. During an observation period of 4 hours, the total number of faeces which were excreted by the animals was recorded. The total number of diarrheal faeces of the control group was considered 100%. Percent inhibition (PI) in defecation was calculated using the following formula: PI=Meandefecation(Controlgroup−Treatedgroup)×100MeanDefecationofControlgroup%
In vitro anthelminthic activity test (Haque MA, 2015): Preparation of extract solution
100mg extract (D. stipulacea) of each was suspended in 10ml distilled water and the suspension was shaken vigorously on a vortex mixture. The suspension was kept overnight at room temperature to solubilize the water soluble part of the extract in aqueous medium and sediment the water insoluble water. After that supernatant aqueous part was separated through a paper filter (Whatman No.1). This extract solution was ready to use for in vitro antihelminthic activity study. Different concentration of each extract solutions (mentioned amount in result) were prepared for the experimental analysis.
Application of extract solution to the black worms (tubifex)
In each test tube approximately 10-12 Panagrellusredivivus (tubifex worms) taken and approximately 2 mL of extract solution of different concentration were given in each test tube. Then the starting time of dosing, time for paralysis and death time of the worm were noted carefully. Two control groups were used in this study to validate the test method and results obtained due to the activity of the test agent. In case of negative control test, only distilled water was added in Petridish containing 10-12 Panagrellusredivivus. No extract was added to prepare control solution. In case of positive control test, Levamisole syrup was used at different concentration mentioned at Table 3 and careful observation was made to see the paralyzing time and death time of Panagrellusredivivus. The time was noted for calculating results.
Statistical analysis
The data was analyzed by one-way ANOVA followed by Dunnett’s test to estimate significant differences between the test and control groups with GraphPad Prism Data Editor for Windows, Version 6.0 (Graph- Pad software Inc., San Diego, CA). Values were expressed as mean ± Standard error of mean (±SEM). P < 0.05-0.001 were considered as statistically significant.
Results
Result of Anti-diarrheal activity
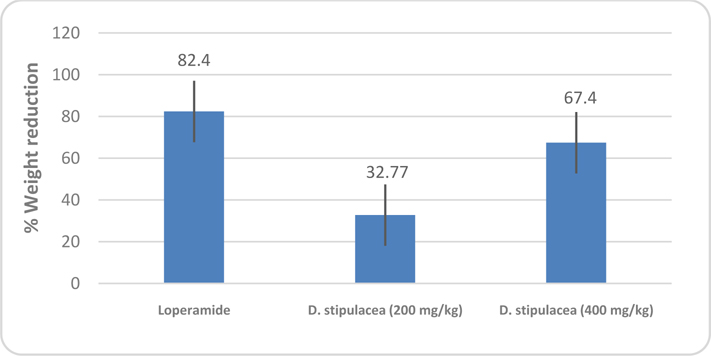
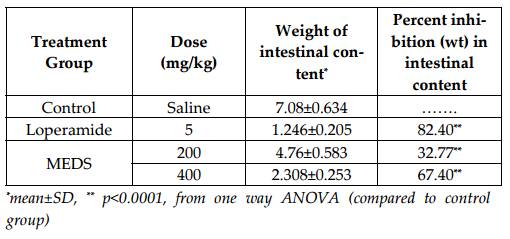
Methanolic extract of D. stipulacea in the investigation of anti-diarrheal activity results in manner of dose dependency. It inhibited the frequency of defecation, induced by castor oil, 42.22% at the dose of 400 mg/kg. The comparison and effectiveness of D. stipulacea described in Figure 1 of Table 1 and Figure 2 of Table 2 . It should also be noted down that the accumulation of intestinal content, induced by castor oil, significantly inhibited by extract, 67.4% (P<0.0001, in contrast to control in one way ANOVA) at 400 mg/kg dose.
Result of Antihelminthic activity
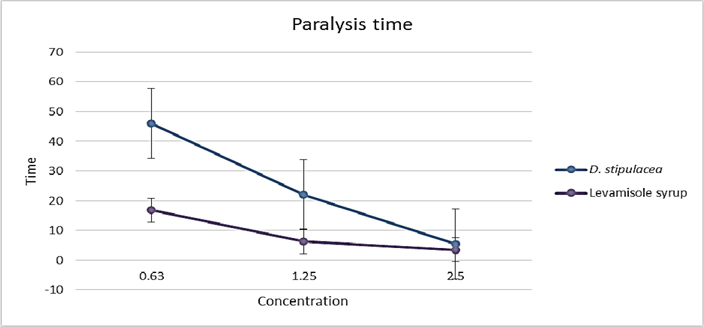
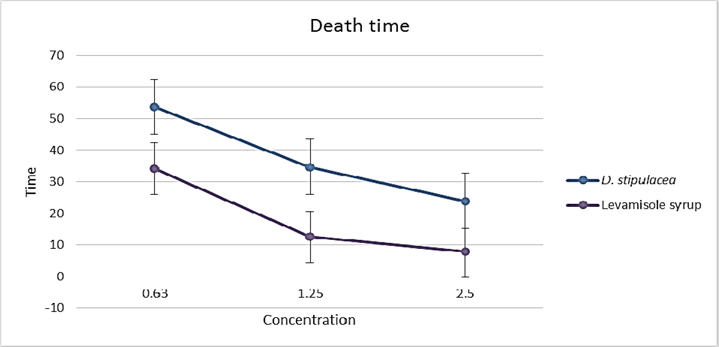
D. stipulacea produced potent effect against P. redivivus (test animal), the efficacy found was concentration dependant. That means as the dose increased the result was close to compared standard. P. redivivus was paralyzed in 5.5 ± 1.323 min ( Table 3 , Figure 3 ) when treated with the dose of 2.5mg and dead in 23.93 ± 1.901 min ( Table 3 , Figure 4 ).
Phytochemical analysis
D. stipulacea leaves extract contains different chemical compounds such as carbohydrate, cholesterol, alkaloid, tannin, cardiac glycoside, saponin, phenol and absence of Steroid and Resin ( Table 4 ) etc.
Discussion
Anti-diarrheal activity
Diarrhea caused due to castor oil induction is ci thcr by irritation and inflammation of mucosa because of ricinoleic acid from castor oil or by the ability of castor oil to increase the volume of intestinal content since it can prevent the reabsorption of NaCl and water. Our extract not only shows effective inhibition of diarrhea induced by castor oil but also prevents the volume of intestinal content, may be, by increasing reabsorption of NaCl and water. It also may be due to inhibition of protein dcnahlration and then reducing inflammation caused by ricinoleic acid in intestine.
Despite the multiplicity of etiologies of diarrhea, literahlres state that there are four major pathophysiologies that lead to diarrhea. These include increased luminal osmolarity (osmotic diarrhea), increased electrolytes secretion (secretory diarrhea), decreased electrolytes absorption, and deranged intestinal motility causing a decreased transit time Agbor et aI., 2004. As an intervention of diarrhea, many antidiarrheal agents elicit effects by reducing the gastrointestinal motility and/or the secretions Gutierrez SP, 2013. In contrast, laxatives and diarrhea causing agents enhance gastrointestinal motility and/or secretions. For instance, castor oil; which is used as an inducer of diarrhca in this Shldy, is known for its laxative effects because of the active principle, retinoic acid. The active principle of castor oil is known to change the electrolyte permeability of the intestinal membrane and through elevated prostaglandin biosynthesis and release it causes diarrhea similar to pathophysiologic conditions that cause diarrhea Besra et al., 2002Brijesh et al., 2009. Because of this, castor oil was used to induce diarrhea in the experimental animals of this study. Different researches have shown that castor oil causes diarrhea 1–2 hours just after administration of 0.1-0.3 ml for mice Rouf et al., 2003. In our experiment diarrhea response was seen within 1 h in most of the experimental subjects because of the high dose of castor oil (0.5 ml/mice). Only those mice that showed the diarrheal response were selected for the experiment, to evaluate the effects of methanolic leaf extract.
Castor oil induced diarrhea is a good model for estimation of diarrheal activity; because it allows observing measurable changes occur in animal model (rat or mice). The extract remarkably reduced stool and also significantly reduced volume and weight of intestinal content. This result signifies both of the model and extract.
Anti-helminthic activity
Our result justifies the hypothesis that we made before starting this experiment. However,this result is not sufficient to make a decision. Even though the antihelminthic potency of this plant is clear but we still need to find the exact mechanism and the causative agent for final decision over this plant part. One probable mechanism of this parasitic inhibition is the interaction between glycogen synthesis and activation of depolarization by activating cholinergic receptors.
The methanolic extract of D. stipulacea demonstrated paralysis as well as death of worms in a less time as compared to piperazine citrate especially at higher concentration of 400 mg/ml. Phytochemical analysis of the crude extracts revealed presence of flavonoids as one of the chemical constituent. Polyphenolic compounds show anthelmintic activity Bate-Smith, 1962. Some synthetic phenolic anthelmintics e.g. niclosamide, oxyclozanide and bithionol are shown to interfere with energy generation in helminth parasites by uncoupling oxidative phosphorylation Martin,1997. It is possible that phenolic content in the extracts of D. stipulacea produced similar effects.
Conclusion
In the above study, we got a remarkable result which is suggesting significant anti-diarrheal and anthelmintic property of D. stipulacea leaves extract. Our extract proved its efficiency in preventing secretory and functional diarrheas even though the mechanism of action in the reduction of diarrhea induced by castor oil is yet not clear. Further study is needed to specify its use in diarrhea like functional, radiational or diarrhea induced by V.cholerae, E. coli. The studied plant part is effectiveto paralyze P. redivivus worms. In terms of time it shows close potency than established anthelmintic drug (Levamisole). Further, in vivo study is required to calculate and establish dose as new lead compound for anthelmintic agent.
Ethical Approval
We took our ethical consent from icddr’b (International Centre for Diarrheal Disease Research, Bangladesh) during collection of anti-diarrheal model and the study protocol was approved by the P&D Committee, Department of Pharmacy, International Islamic University, Chittagong, Bangladesh.
References
-
G.
AG,
LS
Goodman,
A
Gilman.
The pharmacological basisof therapeutics. New York, MacMillan Publishing Co.
1980
.
-
G.A.
Agbor,
T.
Léopold,
N.Y.
Jeanne.
The antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytotherapy research.
2004;
18
:
873-876
.
-
M.
Asif,
A.
Kumar.
Anti-inflammatory activity of ethanolic extract of Dalbergia sissoo (roxb.) Bark.. Malaysian Journalof Pharmaceutical Sciences.
2009;
7
:
39-50
.
-
M.
Asif,
A.
Kumar.
Phytochemical investigation and evaluation of antinociceptive activity of ethanolic extract of Dalbergia sissoo (Roxb.) bark.. Journal of natural science, biology, andmedicine.
2011;
2
:
7-6
.
-
E.
Bate-Smith.
The phenolic constituents of plants and their taxonomic significance. I. Dicotyledons. Journal of the LinneanSociety of London, Botany.
1962;
58
:
95-173
.
-
S.
Besra,
A.
Gomes,
L.
Chaudhury,
J.
Vedasiromoni,
D.
Ganguly.
Antidiarrhoeal activity of seed extract of Albizzia lebbeck Benth. Phytotherapy Research.
2002;
16
:
529-533
.
-
C.
Boschi-Pinto,
L.
Velebit,
K.
Shibuya.
Estimating child mortality due to diarrhoea in developing countries. Bulletin of the World Health Organization.
2008;
86
:
710-717
.
-
K.R.
Brain,
T.D.
Turner.
The practical evaluation of phytopharmaceuticals, Vol 1. Wright-Scientechnica Bristol.
1975
.
-
S.
Brijesh,
P.
Daswani,
P.
Tetali,
N.
Antia,
T.
Birdi.
Studies on the antidiarrhoeal activity of Aegle marmelos unripe fruit: Validating its traditional usage. BMC complementary and alternative medicine.
2009;
9
:
4-7
.
-
Z.-J.
Cheng,
S.-C.
Kuo,
S.-C.
Chan,
F.-N.
Ko,
C.-M.
Teng.
Antioxidant properties of butein isolated from Dalbergia odorifera. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism.
1998;
1392
:
291-299
.
-
R.H.
COTA,
D.M.
GRASSI-KASSISSE,
R.C.
SPADARI-BRATFISCH,
A.R.S.
BRITO.
Anti-ulcerogenic Mechanisms of a Lyophilized Aqueous Extract of Dalbergia monetaria L. in Rats, Mice and Guinea-pigs.. Journal of pharmacy and pharmacology.
1999;
51
:
735-740
.
-
D.W.T.
CROMPTON.
The public health importance of hookworm disease. Parasitology.
2000;
121
:
S39-S50
.
-
P.
Dixit,
R.
Chillara,
V.
Khedgikar,
J.
Gautam,
P.
Kushwaha,
A.
Kumar,
D.
Singh,
R.
Trivedi,
R.
Maurya.
Constituents of Dalbergia sissoo Roxb. leaves with osteogenic activity. Bioorganic & medicinal chemistry letters.
2012;
22
:
890-897
.
-
M.
Dreyfuss,
J.
Shrestha,
S.
Khatry,
E.
Pradhan,
R.
Stoltzfus,
M.
Albonico,
L.
Savioli,
K.
West.
Relationship between iron status and helminth infection among pregnant women in Nepal. FASEB JOURNAL (FEDERATION AMER SOC EXP BIOL 9650 ROCKVILLE PIKE, BETHESDA, MD 20814-3998).
1996
.
-
J.
Galvez,
M.
Crespo,
J.
Jimenez,
A.
Suarez,
A.
Zarzuelo.
Antidiarrhoeic activity of quercitrin in mice and rats. Journal of pharmacy and pharmacology.
1993;
45
:
157-159
.
-
J.
Galvez,
A.
Zarzuelo,
M.
Crespo,
M.
Utrilla,
J.
Jimenez,
C.
Spiessens,
P.
de Witte.
Antidiarrhoeic activity of Sclerocarya birrea bark extract and its active tannin constituent in rats. Phytotherapy Research.
1991;
5
:
276-278
.
-
R.B.
Ganga,
K.P.
Madhu,
R.A.
Vijaya.
Investigation of antioxidant and anti-inflammatory activity of leaves of Dalbergia paniculata (Roxb). Asian Pacific journal of tropical medicine.
2012;
5
:
455-458
.
-
M.D.
Gutiérrez SP,
AH
Munive,
AM
Martínez,
CP
González,
ES
Mendoza.
Antidiarrheal activity of 19-deoxyicetexone isolated from Salvia ballotiflora Benth in mice and rats 8895-8905. 2013
.
-
A.
Hall,
K.S.
Anwar,
A.
Tomkins.
Intensity of reinfection with Ascaris lumbricoides and its implications for parasite control. The Lancet.
1992;
339
:
1253-1257
.
-
K.M.
Haque MA,
Chowdhury MIA
Chowdhury KAA.
Phytochemical Investigation and Assessment of Blumea Lacera (Burm. F.). DC. World J Pharm Res.
2015;
4(3)
:
120-130
.
-
J.B.
Harborne.
Phytochemical methods a guide to modern techniques of plant analysis. Springer Science & Business Media.
1998
.
-
M.
Hood,
S.
Tembhurne,
D.
Sakarkar.
Anthelmintic activity of different extracts of Dalbergia sissoo Roxb. on Indian adult earthworms. Der Pharma Chemica.
2011;
3
:
142-146
.
-
F.
IL.
Organic Chemistry. Singapore Publisher Ltd: Longman.
1983;
2
.
-
M.
Inoue,
S.
Nagi,
E.
Chadeka,
F.
Mutungi,
M.
Osada-Oka,
K.
Ono,
T.
Oda,
M.
Tanaka,
Y.
Ozeki,
K.D.J.
Yombo.
Relationship between mycobacterium tuberculosis and hookworm infections among school children in Mbita, Kenya. J Trop Dis.
2013;
1
:
2
.
-
F.
Jalal,
M.
Nesheim,
Z.
Agus,
D.
Sanjur,
J.
Habicht.
Serum retinol concentrations in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. The American journal of clinical nutrition.
1998;
68
:
623-629
.
-
C.S.
Joshi,
E.S.
Priya,
S.
Venkataraman.
Acute and subacute toxicity studies on the polyherbal antidiabetic formulation Diakyur in experimental animal models. Journal of health science.
2007;
53
:
245-249
.
-
S.L.
Jothy,
Z.
Zakaria,
Y.
Chen,
Y.L.
Lau,
L.Y.
Latha,
S.
Sasidharan.
Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules.
2011;
16
:
5268-5282
.
-
M.
Kalaskar,
V.
Divekar,
P.
Chaugule,
S.
Surana,
D.
Baheti.
Studies on anti-diarrheal activity of Dalberjia sissoo Roxb. in experimental animals. Pharmacologyonline.
2010;
1
:
453-457
.
-
C.
Kokate.
Pharmacohnosy. 16th Edn., Nirali Prakasham, Mumbai, India. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficience virus type I (HIV-I) protease. Phytother Res.
2001;
12
:
488-493
.
-
Y.-c.
Kuo,
C.-W.
Chang,
C.-J.
Chen,
T.-E.
Wang,
W.-H.
Chang,
S.-C.
Shih.
Endoscopic diagnosis of hookworm infection that caused anemia in an elderly person. InternationalJournal of Gerontology.
2010;
4
:
199-201
.
-
J.
Martin,
A.
Keymer,
R.
Isherwood,
S.
Wainwright.
The prevalence and intensity of Ascaris lumbricoides infections in Moslem children from northern Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene.
1983;
77
:
702-706
.
-
R.
Martin.
Modes of action of anthelmintic drugs. The Veterinary Journal.
1997;
154
:
11-34
.
-
A.
Mujumdar,
A.
Misar,
A.
Upadhye.
Antidiarrhoeal activity of ethanol extract of the bark of Dalbergia lanceolaria. Journal of ethnopharmacology.
2005;
102
:
213-216
.
-
OECD Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. OECD Publishing.
.
-
K.
Paech,
M.V.
Tracey.
Modern Methods of Plant Analysis. Vol. III. 1955
.
-
D.
Pritchard,
R.
Quinnell,
M.
Moustafa,
P.G.
McKean,
A.
Slater,
A.
Raiko,
D.
Dale,
A.
Keymer.
Hookworm (Necator americanus) infection and storage iron depletion. Transactions of the Royal Society of Tropical Medicine and Hygiene.
1991;
85
:
235-238
.
-
A.
Rouf,
M.
Islam,
M.
Rahman.
Evaluation of antidiarrhoeal activity Rumex maritimus root. Journal of ethnopharmacology.
2003;
84
:
307-310
.
-
B.
Sivakumar,
V.
Reddy.
Absorption of vitamin A in children with ascariasis. The Journal of tropical medicine and hygiene.
1975;
78
:
114-115
.
-
R.J.
Stoltzfus,
H.M.
Chwaya,
J.M.
Tielsch,
K.J.
Schulze,
M.
Albonico,
L.
Savioli.
Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. The American journal of clinical nutrition.
1997;
65
:
153-159
.
-
M.
Zimmermann.
Ethical guidelines for investigations of experimental pain in conscious animals. Pain.
1983;
16
:
109-110
.
Comments

Downloads
Article Details
Volume & Issue : Vol 2 No 12 (2015)
Page No.: 426-434
Published on: 2015-12-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4932 times
- Download PDF downloaded - 1132 times
- View Article downloaded - 7 times
 Biomedpress
Biomedpress